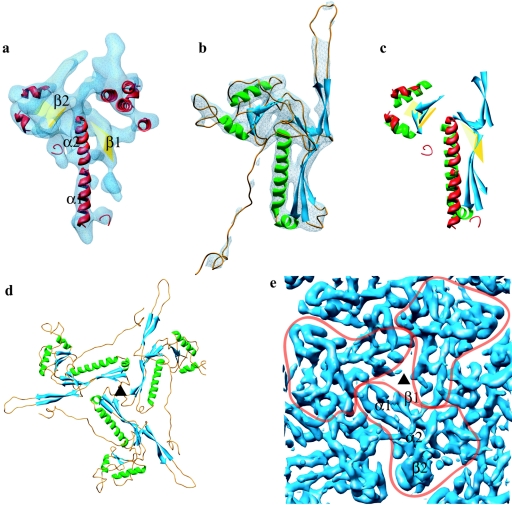
FIG. 2.
Match of the secondary structure elements of HSV-1 capsid VP5 and HK97 phage gp5 and their molecular interactions in the capsids. (a) The isolated VP5 floor domain, in blue, viewed from outside the capsid. SSEhunter identified two long α-helices (red; α1 and α2) adjacent to a large β-sheet (yellow; β1) in the floor domain, as well as a second β-sheet (yellow; β2) and several smaller helices flanking α1 and α2. (b) The HK97 capsid protein (gp5) shown in the same view reveals a similar structural motif. A simulated density map for gp5 at approximately 8-Å resolution is shown in pale blue. (c) Alignment of the secondary structure elements by use of Foldhunter (12) demonstrated a clear match between the floor domain of VP5 and the core structure of gp5. (d) Arrangement of gp5 subunits around a local three-fold axis (▴) in the HK97 capsid as viewed from inside the capsid. (e) Organization of the HSV-1 capsid floor as shown in the same view in panel d. Individual VP5 subunit floor domains are demarcated with the long α-helices (α1, α2) and associated β-sheets (β1, β2) annotated in one subunit.