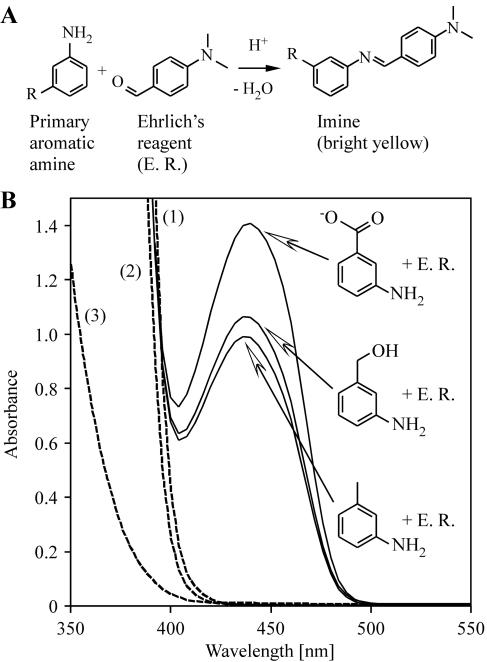
FIG. 1.
Detection of aromatic amines by Ehrlich's reagent. (A) Primary aromatic amines nonspecifically react with p-dimethylaminobenzaldehyde in acidic solution (Ehrlich's reagent) to form yellow imines (28). (B) Spectroscopic detection of imines derived from m-toluidine, m-aminobenzyl alcohol, and m-aminobenzoic acid (1.0 mM each) by Ehrlich's reagent (E. R.) in aqueous solution (with absorption maxima at 436 nm). Controls: 1, mixture of all nitroaromatics (1.0 mM each) with Ehrlich's reagent; 2, Ehrlich's reagent alone; 3, mixture of all nitroaromatics and aromatic amines tested (1.0 mM each) without Ehrlich's reagent.