Abstract
Beginning in mid-2002, a large tuberculosis outbreak occurred among homeless persons in King County, Washington. In order to further monitor the outbreak following its peak in 2003, Mycobacterium tuberculosis isolates from all new King County tuberculosis (TB) patients in 2004 and the first half of 2005 (n = 220) were genotyped by using a rapid comparative genomics-based (genomic deletion-typing) approach, with confirmation by mycobacterial interspersed repetitive units and repetitive-sequence-based PCR (rep-PCR). Results were compared to retrospective genotypic data from 1995 to 2003. The outbreak strain SBRI9, which was not seen among King County homeless persons prior to 2002, accounted for 16 out of 30 TB cases (53%) within this population in 2002. This trend continued with 27 out of 35 cases (77%) caused by the outbreak strain in 2003, 11 out of 13 cases (85%) caused by the outbreak strain in 2004, and 4 out of 10 cases (40%) caused by the outbreak strain in the first 5 months of 2005. Thus, the outbreak strain remained well established within this homeless population throughout the study period. At least four SBRI9 cases were in people who had previously been infected by other strains. The novel PCR-based strain-typing approach used in this investigation proved to be cost-effective and very rapid. In most cases, it was possible to analyze DNA extracted directly from primary isolation (Mycobacterium growth indicator tube) cultures submitted by clinical laboratories, a feature that markedly reduced the delay between diagnosis and strain typing results. This rapid turnaround facilitated public health efforts to prevent new outbreaks involving this strain.
In 2002, public health officials detected a 100% increase in tuberculosis (TB) among homeless people in Seattle. Genotypic strain typing of Mycobacterium tuberculosis isolates identified an outbreak cluster involving a pansusceptible strain designated SBRI9 (13). This strain had also caused case clusters in New York, New Jersey, and Michigan (13). The first King County cases were diagnosed in May 2002, and by the end of 2003, the outbreak exceeded 48 cases. Most transmission occurred in facilities and social centers catering to the homeless, with only a few cases occurring outside of the homeless community.
An aggressive public health response substantially curbed the outbreak by the end of 2003 (K. L. Lofy et al., submitted for publication). Nevertheless, it was deemed useful to continue to monitor the transmission of strain SBRI9 in 2004 and beyond for two reasons. First, the Centers for Disease Control and Prevention estimated that at least 1,000 persons were exposed to the outbreak strain in 2002-2003, mostly in Seattle homeless facilities. Tuberculin skin test positivity among people who spent nights at these facilities was >4-fold greater than the baseline level for this population (Lofy et al., submitted). Due to the difficulties associated with monitoring the treatment of homeless people, few of the exposed individuals with positive tuberculin skin tests received treatment for latent tuberculosis. This left within the community a reservoir of people who are infected with the outbreak strain and at risk of starting new outbreaks. This risk was addressed in part by continuing to track the transmission of the outbreak strain within the community.
A second incentive for ongoing monitoring of SBRI9 transmission arose from genotypic analysis, which placed the strain into the N subfamily of the pandemic W-Beijing family of M. tuberculosis isolates (13). The Beijing family is one of several phylogenetic clades of M. tuberculosis that are thought to account for disproportionately large numbers of TB case clusters worldwide (2, 3, 6, 14). Beijing strains may have innate characteristics that favor clonal expansion within human populations. In nonhuman models of tuberculosis infection, Beijing strains have hypervirulent phenotypes and elicit altered cytokine responses relative to control strains (11, 12, 21). Specific genetic and phenotypic properties which might account for these observations have been described previously (16, 17). Localized studies have shown that Beijing family strains can spread aggressively within some populations (1, 4). However, it remains unclear whether the Beijing family is truly expanding globally, as the global abundance of certain phylogenetic clades of M. tuberculosis could alternatively be explained by stable association with human populations that have themselves expanded (8). Despite these uncertainties, the introduction of a new Beijing family strain into a population is potentially significant from the epidemiological and public health standpoints.
Standard M. tuberculosis strain fingerprinting methods, including spoligotyping, IS6110-targeted restriction fragment length polymorphism (IS6110-RFLP) analysis, mycobacterial interspersed repetitive unit (MIRU) analysis, and repetitive-sequence-based PCR (rep-PCR) (5, 13, 18), are too time consuming or expensive for a postoutbreak investigation aimed at diagnosing new cases involving a single genotype. Therefore, a new approach was designed that took advantage of the expanding M. tuberculosis comparative genomic databases in the literature. This information was used to generate a PCR-based test for strain SBRI9 based on genomic deletion typing (deligotyping). This approach allowed us to identify new SBRI9 cases in a very rapid and cost-effective fashion and to observe the clonal expansion of this strain within this homeless population.
MATERIALS AND METHODS
Identification of genomic deletions specific for SBRI9.
Genomic deletions in strain SBRI9 relative to M. tuberculosis reference strains H37Rv and CDC1551 were identified by Affymetrix GeneChip analysis following protocols described previously (9, 20). Briefly, genomic DNA purified from a reference isolate of strain SBRI9 was digested and hybridized onto the Affymetrix M. tuberculosis GeneChip. Hybridization signal intensity was analyzed with DELSCAN software (AbaSci, San Pablo, CA) and the Affymetrix Microarray Suite to identify DNA segments likely to be deleted relative to those from H37Rv. Deletions were confirmed by PCR and sequencing. Those deletions that were specifically characteristic of strain SBRI9 were identified by comparison to genomic deletion databases (9, 20).
PCR-based deligotyping.
For each of the two deletions predicted to be specific for strain SBRI9, a multiplex PCR was designed to amplify either the deleted or nondeleted genomic region. Two external primers (forward and reverse) and one internal primer were used per locus. If a region was deleted, the two external primers yielded a PCR amplification product of a predicted size. If the region was not deleted, the external primers did not yield a product due to the length of intervening sequence. Instead, one external primer combined with the internal primer to yield a distinct product (Table 1). PCR conditions and retrospective validation of the test are described in Results.
TABLE 1.
Deligotyping primers
| Deletion | Primer sequence
|
Predicted PCR amplification product size (bp)
|
|||
|---|---|---|---|---|---|
| Forward | Reverse | Internal | Deleted (forward and reverse primers) | Nondeleted (forward and internal primers) | |
| RD168BW | TTCGTCGTGGACGCGTCGGGCAAGAACA | ACGTTGCGCTGCGCGGTAGACCTCATCAATT | GACGACGGCCAAGAATTCCCGGTACGCGT | ∼1,200 | 767 |
| RD200BW | AGGTCGAATCCATAACGCCGCGCCAGCAT | CGGTAGCTGCGTGGCGCACCACGA | CACGGAACAGACCACATGGCGCTCGC | ∼900 | 535 |
MIRU strain typing.
MIRU analysis was one of two methods used for confirmatory strain typing. PCR primers and cycling conditions were as described by Supply et al. (18), except that amplification of a subset of MIRU loci (numbers 16, 23, 24, 26, 27, and 39) was conducted in the presence of 1.5 mM Mg2+ and 1× Resolution Solution (Roche Molecular). Other MIRU loci were amplified using the previously described PCR mix (18). Locus 20 failed to amplify consistently well under any conditions and was excluded from the MIRU panel. Samples were visualized on a 2% agarose gel by using both a 1-kb Plus ladder (Invitrogen) and a 50-bp step ladder (Roche Molecular) for sizing. H37Rv and SBRI9 DNAs were used as controls.
rep-PCR.
The DiversiLab system (Bacterial Barcodes, Inc., Houston, TX), which exhibits resolving power equivalent to that of IS6110-RFLP, was the second confirmatory strain-typing method and was applied as described previously (5). Briefly, genomic DNA was amplified using a mycobacterial kit. The amplicons were analyzed on the system's microfluidic chips by using an Agilent 2100 Bioanalyzer. H37Rv and SBRI9 DNAs were used as controls. A cutoff value of 93% similarity (5) was used to establish strain identity.
Prospective deligotyping of new M. tuberculosis isolates.
M. tuberculosis isolates (one per patient) collected by the Seattle-King County TB Control Program were submitted to the Seattle Biomedical Research Institute (SBRI) for strain typing. Submissions came in the form of 1-ml aliquots taken directly from positive Mycobacterium growth indicator tube (MGIT) cultures. DNA was extracted using the MoBio UltraClean Microbial DNA isolation kit (MoBio, Inc.) with protocol modifications for Mycobacterium spp. An average of 200 ng genomic DNA was obtained per sample. Immediately prior to extraction, the samples were also subcultured for archiving and for confirmatory analysis if needed.
For each of the 220 isolates received, a combination of three PCRs was used to presumptively identify SBRI9 isolates. One of the reactions was a deligotyping test consisting of a multiplex PCR for deletions RD168BW and RD200BW. The other two were partial MIRU analyses for locus 23, where SBRI9 exhibits an unusual single-repeat pattern, and locus 10. Samples that were consistent with SBRI9 by deligotyping (those with deletions DS168BW and DS200BW) and partial MIRU (loci 10 and 23) were subjected to confirmatory analysis in the form of an expanded MIRU panel and rep-PCR.
Retrospective genotypic data.
M. tuberculosis isolates from homeless patients in 1995-1996 and 1998-2000 had been characterized for previous, unpublished studies by spoligotyping and IS6110 restriction fragment length polymorphism. Isolates from 2001 were typed by the Centers for Disease Control and Prevention as part of the SBRI9 outbreak investigation (Lofy et al., submitted).
Definitions.
The Seattle-King County TB Control Program defines as “homeless” people who were homeless at the time of diagnosis, as well as people who have a significant history of homelessness. This definition might encompass people who lived in subsidized or transitional housing at the time of diagnosis but who also spent time in places where homeless people congregate, such as shelters, sobering centers, and specific park locations.
Human subjects.
Data reported in this article were collected during the course of a tuberculosis outbreak investigation by the Seattle-King County Department of Public Health. The Human Subjects Protection Institutional Review Board at SBRI determined that the work was exempt from human subjects institutional review board review because M. tuberculosis isolates were collected for purposes other than research and because donors were anonymous.
RESULTS
Rationale for postoutbreak monitoring.
The 2002-2003 tuberculosis outbreak among homeless people in Seattle has been described by Milan et al. (13) and Lofy et al. (submitted). The outbreak strain SBRI9 was thought to have been introduced to Seattle sometime around 2001, and the first cases were diagnosed in May 2002. Of 48 confirmed outbreak cases in 2002 and 2003, 44 (92%) were in homeless people. Forty-five (94%) were in people born in the United States, consistent with the overall makeup of the Seattle homeless population, and 7 (15%) were human immunodeficiency virus positive. In Seattle in recent years, 11% to 23% of TB patients were homeless. Outside of the homeless population, most TB cases in Seattle occur in foreign-born people (65% to 79% of total annual cases) and are thought to be reactivations of infections acquired in home countries (http://www.metrokc.gov/health/tb/tb2003.pdf). Because the epidemiologies of TB for these two groups differ, and because neither of two recent TB outbreaks in 1998-1999 and 2002-2003 spread beyond the homeless population in King County, this report focuses on the homeless as a distinct population.
Most transmission in the 2002-2003 outbreak occurred in a few homeless facilities (Lofy et al., submitted). Few of the >1,000 people who were exposed to strain SBRI9 at these and other sites received treatment for latent tuberculosis. This left within the community a pool of people who are at risk of starting new outbreaks. In order to identify new SBRI9 cases as quickly as possible following diagnosis, a strain-targeted PCR-based screen was designed to rapidly and inexpensively identify new SBRI9 isolates from all new TB cases in Seattle in 2004.
Characterization of genomic deletions in SBRI9.
Affymetrix GeneChip analysis identified 12 genomic regions that were deleted from strain SBRI9 relative to reference genome sequences strains. Ten of them have been described (7, 19). The deletions ranged in size from approximately 1 to 12 kb and affected from 1 to 16 open reading frames each. Eight deletions found in strain SBRI9, designated RD105, RD149, RD152, RD181, RD168BW, RD200BW, RD224c, and RD207, have been observed in various combinations in other Beijing family strains (19). Only one other Beijing family strain, designated TN1595, had all eight of these deletions (19). Strain TN1595 was involved in a nosocomial MDR-TB outbreak in New York City in 1989-1990 (10), and it has the “N2” IS6110-RFLP pattern that was reported previously to be 94% similar to that of strain SBRI9 (13). Therefore, the genotypic linkage identified by deligotyping was in concordance with IS6110-RFLP results.
The combination of two deletions, RD168BW and RD200BW, was not seen in any strain other than SBRI9 and TN1595 in databases of clinical isolates that had been deligotyped in similar fashion (19, 20). Therefore, these two deletions were used for rapid PCR-based identification of strain SBRI9.
Retrospective validation of a multiplex PCR for specific detection of the SBRI9 deligotype.
For each of the two deletions in Table 1, a set of forward, reverse, and internal primers was combined into a multiplex PCR that yielded readily distinguishable bands indicating the deletion or nondeletion of each locus (Table 1). The specificity of this test for strain SBRI9 was evaluated in a retrospective analysis of 135 archived M. tuberculosis isolates from Seattle-King County. Strains within the sample set had previously been genotyped by spoligotyping and IS6110-RFLP (13). Both deletions were detected in all 14 SBRI9 isolates within the sample set, and neither was found in any of the 121 non-SBRI9 isolates within the sample set.
Prospective deligotyping to identify new SBRI9 isolates.
For a 19-month period beginning in November 2003, samples taken directly from positive MGIT cultures in two clinical microbiology labs were transferred to SBRI for analysis. In total, 220 isolates were analyzed, accounting for all known tuberculosis cases within the county during the period. The sample included 22 isolates from homeless people, a number that constituted 100% of known culture-positive homeless cases within the county during the period (a 23rd case was culture negative). PCR analysis of DNA extracted directly from MGIT samples bypassed the need for culture amplification of isolates, reducing by days or weeks the time lag between diagnosis and strain identification.
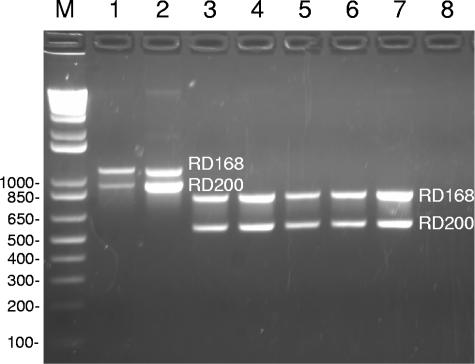
DNA extracted from each MGIT sample was subjected to three PCRs: the multiplex PCR for RD168BW and RD200BW (Fig. 1) and a separate PCR for each of the two most distinctive MIRU loci. When results of all three tests were consistent with the SBRI9 genotype, the TB Control Program was notified of the presumptive identification of an SBRI9 isolate. This normally occurred within 2 days of receipt of a MGIT sample. Seventeen of the 220 samples analyzed yielded positive results in all three tests. In all cases, these strains were subsequently confirmed to be SBRI9 by analysis of nine additional MIRU loci and by rep-PCR (5). Thus, relative to high-resolution standards, deligotyping exhibited 100% sensitivity for the SBRI9 genotype.
FIG. 1.
Deligotyping by multiplex PCR for the RD168BW and RD200BW deletions. Lane M, 1-kb Plus DNA ladder. Lane 1, control DNA from a reference isolate of strain SBRI9, showing PCR product sizes expected from an isolate with deletions at both positions. Lanes 2 to 7, clinical submissions. The isolate in lane 2 exhibited the deleted genotype at both positions, while the others exhibited the nondeleted genotype at both positions. Lane 8, negative control (water). The isolates in lanes 1 and 2 also exhibited the SBRI9 genotype at MIRU loci 23 and 10, respectively, whereas the other isolates exhibited the SBRI9 genotype at neither MIRU loci (data not shown). Deligotyping reactions used a PCR mix of 10× PCR buffer (Invitrogen), 200 μM each dNTPs (Roche Molecular), 1.5 mM Mg2+, 100 nM each primer, 2.5 U Taq polymerase (Invitrogen), and 25 ng template. Cycling conditions were 95°C for 5 min, 35 cycles of 95°C for 1 min, 62°C for 1 min, and 72°C for 2 min, with a final extension at 72°C for 5 min. PCR products were visualized on a 1.5% agarose gel.
The 17 new SBRI9 isolates raised to at least 65 the total number of cases associated with this cluster. The remaining 203 isolates analyzed were not consistent with the SBRI9 genotype by any of the deligotyping (deletions RD168BW and RD200BW) and partial MIRU (loci 10 and 23) tests and were not analyzed further. Fifteen of the 17 new SBRI9 isolates were from homeless people.
Expansion of the SBRI9 genotype within the homeless community.
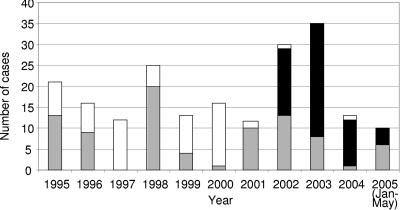
In order to assess the extent to which strain SBRI9 has expanded within the Seattle homeless community, we compared 2003-2004 genotypic data to retrospective data on tuberculosis among Seattle homeless people in the previous 8 years. Homeless cases numbered between 12 and 21 per year throughout the period, except during the 2002-2003 outbreak and during a smaller outbreak in 1998-1999 (Fig. 2). Genotypic data were available for 62 out of 120 cases that occurred in Seattle homeless people during the period from 1995 through April 2002, immediately prior to the diagnosis of the first SBRI9 case in May 2002. No data or archived bacterial isolates were available for the other 58 cases (Fig. 2).
FIG. 2.
Genotypes of M. tuberculosis isolates from King County homeless patients in 1995-2004. Cases associated with the SBRI9 genotype (black bars), with all other genotypes (gray bars), and with no genotypic information (white bars) are indicated. As discussed in the text, it is assumed that few or none of the untyped isolates were SBRI9 and that these isolates were genotypically diverse like the typed isolates from each year.
Previously unpublished genotypic analysis of 1995-1996 homeless isolates revealed extensive genotypic diversity, with no clusters larger than three cases and no isolates with the SBRI9 genotype. Twenty isolates were tested over the course of the 1998-1999 outbreak investigation. Other than the 1998-1999 outbreak cluster (10 cases), no clusters of two or more cases were observed. The 1998-1999 outbreak strain had a three-band IS6110-RFLP pattern and therefore was unrelated to the 15-band SBRI9 genotype. One case involving this strain was diagnosed in 1999, two were diagnosed in 2001, and none were diagnosed thereafter. In 2001, there were 12 homeless people with cases of TB, and 10 of these cases were genotyped and found to be diverse, with no SBRI9 cases and no cluster larger than 3 patients diagnosed (Lofy et al., submitted). Similar diversity was seen among the five cases diagnosed between January 2002 and April 2002 (not shown). Thus, from January 1995 through April 2002, there was a consistent background incidence of 12 to 25 genotypically diverse TB cases per year within the King County homeless population. Genotypic data were not available for a significant fraction of cases during this period (Fig. 2); however, there were no epidemiological indications of case clusters among the untyped cases.
Strain SBRI9 is believed to have been introduced to the area shortly before the diagnosis of the first outbreak case in May 2002 (Lofy et al., submitted). The ensuing outbreak increased the annual number of tuberculosis cases within the population. The number returned to a more typical 13 cases in 2004. However, the epidemiology of TB within the community was altered in the process, most notably by the establishment of strain SBRI9 as the dominant clone within the population during the period of the study (Fig. 2).
Exogenous reinfection by strain SBRI9 of people previously infected by other strains.
Examination of coded patient records by the Seattle-King County TB control program revealed that four SBRI9 cases from 2002-2003 were exogenous reinfections. During the time period 1996-1999, each of these four patients had been diagnosed and treated for tuberculosis caused by a strain other than SBRI9. Only one of the four reinfected patients was human immunodeficiency virus positive.
DISCUSSION
Deligotyping by PCR was used to identify new SBRI9 cases as quickly as possible following diagnosis. The information it generated enabled the TB Control Program to focus its investigative resources on the cases deemed likely to cause additional clusters. In most instances, results were available within 1 to 2 days of receipt of a positive MGIT sample. In view of the slow growth rate of the pathogen in culture, the ability to conduct this analysis directly on primary MGIT enrichment samples resulted in significant time savings relative to the traditional practice of typing isolates grown on solidified media. Analysis of primary enrichment samples presents a risk of ambiguous results due to the presence of multiple clones or contaminants within the MGIT cultures. However, such results would have been visible as aberrant or multiple PCR products on deligotyping and MIRU gels, and no such results were observed in this analysis. The availability of genotyping data within 1 or 2 days of diagnosis greatly facilitated the prioritization of tuberculosis control efforts, especially when applied to a homeless population such as this one. This experience demonstrated how newer strain genotyping methods, combined with the expanding global database of M. tuberculosis comparative genomic data, can be applied in real time to benefit TB control. A similar approach was applied to the investigation of a TB outbreak in Great Britain (15).
Deligotyping targeted to a single strain, as described here, is not useful for routine surveillance genotyping. Recently, a filter array-based deligotyping method that does not have this limitation was reported (7). The filter array-based method also bypasses the need for GeneChip analysis. Further improvements will make deligotyping an attractive alternative for genotypic fingerprinting of pathogen strains. It is fast and capable of excellent resolving power when the number of interrogated polymorphisms is increased. The results are highly portable. In addition, because the genetic makeup of deleted regions is known, deligotyping provides useful information on the genomic makeup of fingerprinted strains.
The 2004-2005 genotypic data indicated that the outbreak strain SBRI9 continues to cause disease within this homeless population but has not spread far beyond the homeless community in Seattle. A striking feature of Fig. 2 is the extent to which strain SBRI9 came to dominate TB epidemiology within this population during and after the outbreak. This trend peaked in 2004, when TB was nearly monoclonal within this population. This may have been driven in part by innate characteristics of this Beijing family strain that caused it to spread more aggressively than other strains. For example, strain SBRI9 may have superinfected people who were previously or concurrently infected with other strains. This was observed in at least four cases in the SBRI9 outbreak, and additional heterologous reinfections were likely to have gone undetected due to the lack of patient history or past strain genotype information. This possibility is interesting; however, trends in host or environmental factors may have played equal or greater roles in the success of the outbreak strain. Additional prospective analysis is needed to understand how pathogen, host, and environmental factors contributed to this success.
Acknowledgments
We thank Linda Lake, Paul Swenson, and clinical laboratory personnel at Harborview Medical Center and Public Health Seattle-King County Tuberculosis Control Program. We are also grateful to Kim Field, Joseph Aharchi, and others at the Washington State Department of Health for facilitating the outbreak investigation and to Kathy Lofy at the Centers for Disease Control and Prevention for permitting us to cite her submitted manuscript. Tige Rustad, Tanj Bennett, Natasha Close, and Kathleen Horan provided critical feedback on the manuscript.
This work was supported by Puget Sound Partners for Global Health, Public Health Seattle-King County Tuberculosis Control Program, and SBRI Trustees. Equipment grants from the M. J. Murdock Charitable Trust and the Firland Foundation are gratefully acknowledged.
REFERENCES
- 1.Anh, D. D., M. W. Borgdorff, L. N. Van, N. T. Lan, T. van Gorkom, K. Kremer, and D. van Soolingen. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 6:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, P. F., and M. D. Cave. 2003. Molecular epidemiology of tuberculosis. N. Engl. J. Med. 349:1149-1156. [DOI] [PubMed] [Google Scholar]
- 3.Bifani, P. J., B. Mathema, N. E. Kurepina, and B. N. Kreiswirth. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 10:45-52. [DOI] [PubMed] [Google Scholar]
- 4.Caminero, J. A., M. J. Pena, M. I. Campos-Herrero, J. C. Rodriguez, I. Garcia, P.Cabrera, C. Lafoz, S. Samper, H. Takiff, O. Afonso, J. M. Pavon, M. J. Torres, D. van Soolingen, D. A. Enarson, and C. Martin. 2001. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am. J. Respir. Crit. Care Med. 164:1165-1170. [DOI] [PubMed] [Google Scholar]
- 5.Cangelosi, G. A., R. J. Freeman, K. N. Lewis, D. Livingston-Rosanoff, S. J. Milan, and S. Goldberg. 2004. Evaluation of a high throughput repetitive-sequence-based PCR system for DNA fingerprinting of Mycobacterium tuberculosis and Mycobacterium avium complex. J. Clin. Microbiol. 42:2685-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. A. Dang, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, M. L. Ho, A. Makristathis, C. Mammina, G. Martin, P. Mostrom, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 41:1963-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goguet de la Salmoniere, Y. O., C. C. Kim, A. G. Tsolaki, A. S. Pym, M. S. Siegrist, and P. M. Small. 2004. High-throughput method for detecting genomic-deletion polymorphisms. J. Clin. Microbiol. 42:2913-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsh, A. E., A. G. Tsolaki, K. DeRiemer, M. W. Feldman, and P. M. Small. 2004. Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc. Natl. Acad. Sci. USA 101:4871-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato-Maeda, M., J. T. Rhee, T. R. Gingeras, H. Salamon, J. Drenkow, N. Smittipat, and P. M. Small. 2001. Comparing genomes within the species Mycobacterium tuberculosis. Genome Res. 11:547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurepina, N. E., S. Sreevatsan, B. B. Plikaytis, P. J. Bifani, N. D. Connell, R. J. Donnelly, D. van Soolingen, J. M. Musser, and B. N. Kreiswirth. 1998. Characterization of the phylogenetic distribution and chromosomal insertion sites of five IS6110 elements in Mycobacterium tuberculosis: non-random integration in the dnaA-dnaN region. Tuber. Lung Dis. 79:31-42. [DOI] [PubMed] [Google Scholar]
- 11.Li, Q., C. C. Whalen, J. M. Albert, R. Larkin, L. Zukowski, M. D. Cave, and R. F. Silver. 2002. Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 70:6489-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. USA 98:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milan, S. J., K. A. Hauge, N. E. Kurepina, K. H. Lofy, S. Goldberg, M. Narita, C. Nolan, P. McElroy, B. Kreiswirth, and G. A. Cangelosi. 2004. Expanded geographical distribution of the N family of Mycobacterium tuberculosis strains within the United States. J. Clin. Microbiol. 42:1064-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray, M., and E. Nardell. 2002. Molecular epidemiology of tuberculosis: achievements and challenges to current knowledge. Bull. W. H. O. 80:477-482. [PMC free article] [PubMed] [Google Scholar]
- 15.Rajakumar, K., J. Shafi, R. J. Smith, R. A. Stabler, P. W. Andrew, D. Modha, G. Bryant, P. Monk, J. Hinds, P. D. Butcher, and M. R. Barer. 2004. Use of genome level-informed PCR as a new investigational approach for analysis of outbreak-associated Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 42:1890-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 17.Safi, H., P. F. Barnes, D. L. Lakey, H. Shams, B. Samten, R. Vankayalapati, and S. T. Howard. 2004. IS6110 functions as a mobile, monocyte-activated promoter in Mycobacterium tuberculosis. Mol. Microbiol. 52:999-1012. [DOI] [PubMed] [Google Scholar]
- 18.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsolaki, A. G., S. Gagneux, A. S. Pym, Y. O. Goguet de la Salmoniere, B. N. Krieswirth, D. van Soolingen, and P. M. Small. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsolaki, A. G., A. E. Hirsh, K. DeRiemer, J. A. Enciso, M. Z. Wong, M. Hannan, Y. O. Goguet de la Salmoniere, K. Aman, M. Kato-Maeda, and P. M. Small. 2004. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc. Natl. Acad. Sci. USA 101:4865-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, M., J. Gong, Z. Yang, B. Samten, M. D. Cave, and P. F. Barnes. 1999. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J. Infect. Dis. 179:1217. [DOI] [PubMed] [Google Scholar]