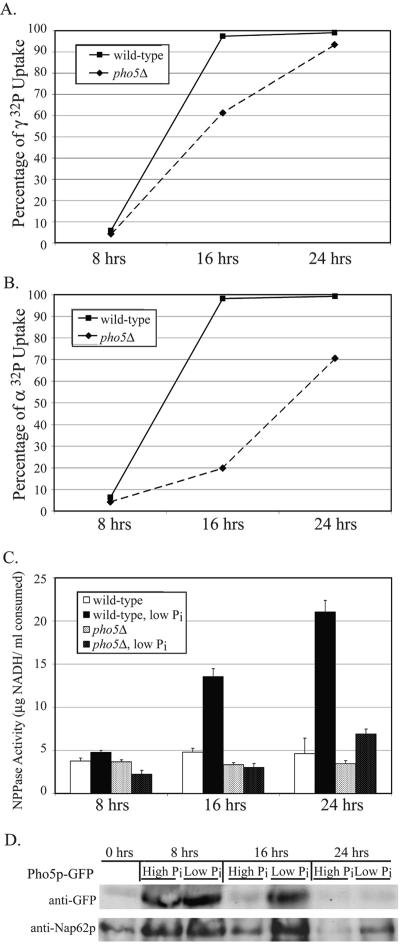
FIG. 5.
Nucleotide phosphate hydrolysis and uptake is also affected by Pho5p, a component of the PHO response. (A) The ability to import extracellular phosphate from nucleotide sources was determined in pho5-null mutants. Cells were grown in low-phosphate medium containing [γ-32P]ATP, and the rate of imported phosphate was compared to a wild-type strain over 24 h. The mutant strain was unable to hydrolyze and subsequently import phosphate as effectively as the wild type. (B) Hydrolysis of [α-32P] from ATP was measured over a 24-h time course. The pho5Δ mutant had a pronounced shortcoming in phosphate hydrolysis, as demonstrated by the lack of imported phosphate at 16 and 24 h, but still less severe than the npp1Δ npp2Δ double mutant. (C) Utilization of ATP as an extracellular phosphate substrate was tested by comparing a pho5Δ mutant to a wild-type strain grown in low-phosphate medium containing ATP. E-NPP activity was measured over a 24-h time course, where utilization of ATP as a substrate for APase activity was measured as a function of oxidized NADH. The lack of activity measured in the pho5Δ mutant demonstrated that ATP is a substrate of Pho5p. (D) Protein expression levels of Pho5p were measured over 24 h from cultures grown in YPD or phosphate-depleted medium. Protein levels were detected by immunoblotting against Pho5p-GFP fusions. Pho5p expression is enhanced during phosphate starvation.