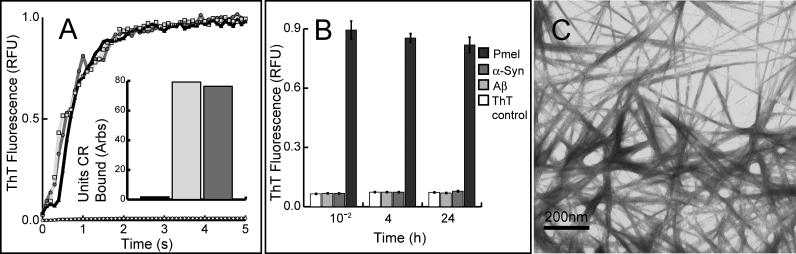
Figure 3. rMα Rapidly Forms Thioflavin T– and Congo Red–Positive Fibers under Nondenaturing Conditions.
(A) rMα samples (in 8 M GdmCl to preserve an unfolded, nonaggregated state) were diluted by manual mixing to start an amyloid fiber formation time course (monitored by thioflavin T fluorescence) at varying pHs: pH 7.4 (black line, triangles), pH 6.0 (dark grey line, circles), and pH 4.85 (light grey line, squares); control (thioflavin T buffer) (black line, white diamonds ). The inset bar graph reflects endpoint Congo red binding of equimolar amounts of deposits of Mα formed at pH 7.4 (dark grey), Aβ 1–40 fibers associated with Alzheimer disease (light grey), and control (Congo red buffer) (black).
(B) rMα (Pmel) forms thioflavin T (ThT)–positive aggregates at least four orders of magnitude faster than either α-synuclein (α-Syn) or Aβ when all three polypeptides are diluted from 8 M GdmCl into physiological buffer (error bars represent the standard deviation of triplicate samples).
(C) Transmission electron micrograph of typical rMα amyloid fibers with an average diameter of 10 nm, formed under nondenaturing conditions.