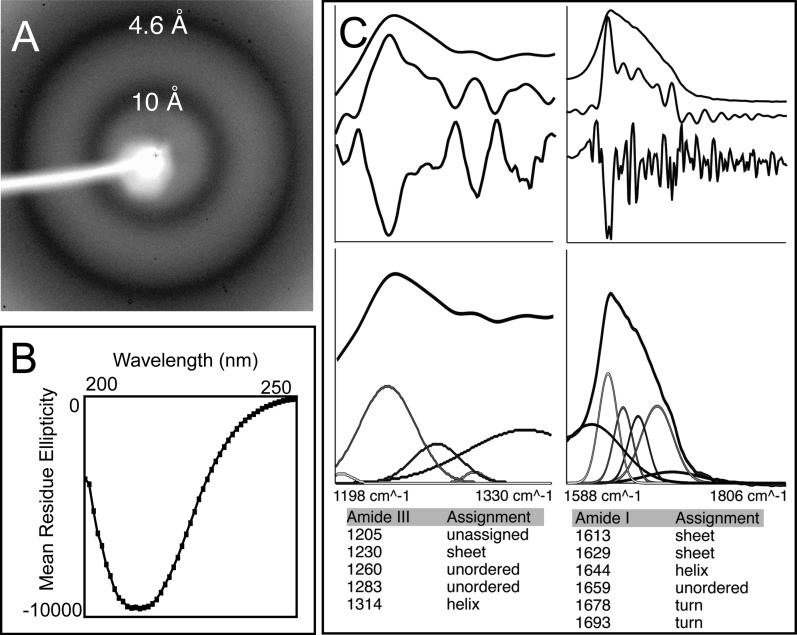
Figure 4. rMα Fibers Have a Cross-β Sheet Structure.
(A) X-ray powder diffraction of lyophilized rMα fibers formed in vitro exhibit a very strong reflection at 4.6 Å and a strong reflection at 10 Å, which is expected of an amyloid cross-β sheet structure.
(B) The far-UV CD spectra of soluble Mα aggregates formed at low concentrations to avoid precipitation support a predominantly β-sheet structure. Mα aggregates are approximately 11% α-helix, 32% β-sheet, 23% β-turn, and 33% disordered, based on curve fitting with a basis set of 43 soluble proteins. Since β-sheet content is estimated using a set of proteins not composed of cross-β sheet structures, the potential error in the estimate cannot be determined.
(C) The attenuated total reflectance FT-IR spectrum of aggregated rMα in the solid state supports a β-sheet-rich structure. Peaks in the amide III (top left, upper curve) and I (top right, upper curve) regions were identified using Fourier self-deconvolution (top left and right, middle curve) and confirmed by second derivative analysis (top left and right, bottom curve). Peak assignments are listed, and were used to fit the original spectrum using fixed Gaussian peaks at the assigned positions (bottom). Peaks assigned to β-sheet regions of the spectrum accounted for a large percentage of the total area in the amide I and III regions.