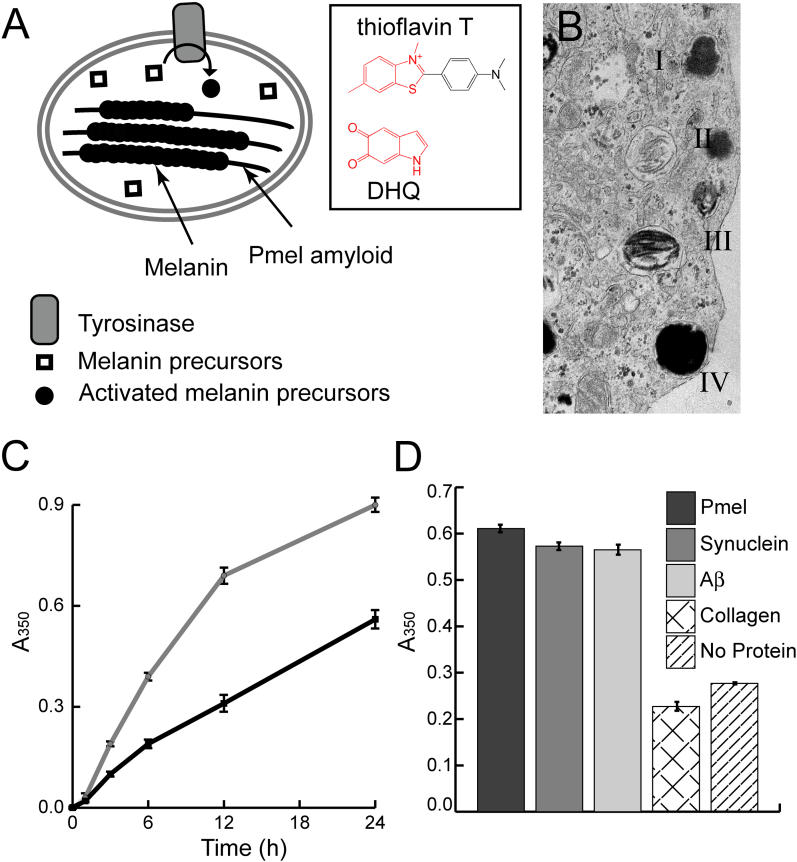
Figure 5. Amyloid, Including rMα, Specifically Accelerates Melanin Synthesis.
(A) In melanosomes, assembly of activated melanin precursors, generated by tyrosinase, occurs along Pmel17 fibers. The boxed portion of (A) illustrates the amyloid-binding dye thioflavin T and the activated melanin precursor DHQ, which possess similar core structures. This suggests an explanation for the ability of Pmel17 to concentrate and organize melanin precursors, thereby enabling melanogenesis.
(B) In vivo, melanosome maturation is a four-step process (I–IV) in which initial formation of the Pmel17 fibrillar matrix (II) enables subsequent melanin polymerization along the Pmel17 fibers (III) (Adapted with permission from [16].)
(C) A time course of melanin synthesis in vitro shows that insoluble rMα amyloid increases the amount of insoluble melanin formed per unit time (grey line) versus a control reaction lacking rMα (black line).
(D) Melanin synthesis after 20 h was also evaluated in the presence of insoluble rMα amyloid, α-synuclein amyloid, Aβ amyloid, and collagen IV α-helical fibers. The melanin precursor D,L-DOPA was incubated in the presence of the enzyme tyrosinase and the amyloid of interest at room temperature. Melanin content of each reaction condition was measured by pelleting insoluble melanin, dissolving it in 1 M NaOH, and measuring the absorbance at 350 nm. Supernatant melanin content was equal for all samples.
In (C) and (D) error bars represent the standard deviation between triplicate samples.