Summary
Telomeres are nucleoprotein complexes at the end of eukaryotic chromosomes, with important roles in the maintenance of genomic stability and in chromosome segregation. Normal somatic cells lose telomeric repeats with each cell division both in vivo and in vitro. To address a potential role of nuclear architecture and epigenetic factors in telomere-length dynamics, the length of the telomeres of the X chromosomes and the autosomes was measured in metaphases from blood lymphocytes of human females of various ages, by quantitative FISH with a peptide nucleic-acid telomeric probe in combination with an X-chromosome centromere–specific probe. The activation status of the X chromosomes was simultaneously visualized with antibodies against acetylated histone H4. We observed an accelerated shortening of telomeric repeats in the inactive X chromosome, which suggests that epigenetic factors modulate not only the length but also the rate of age-associated telomere shortening in human cells in vivo. This is the first evidence to show a differential rate of telomere shortening between and within homologous chromosomes in any species. Our results are also consistent with a causative role of telomere shortening in the well-documented X-chromosome aneuploidy in aging humans.
Introduction
Telomeres are nucleoprotein complexes located at the end of eukaryotic chromosomes. They play important roles in the maintenance of genomic integrity. Since the conventional DNA replication is unable to replicate the very ends of linear chromosomes, normal somatic cells lose telomeric repeats with each cell division, both in vivo and in vitro. The enzyme telomerase, with associated proteins (Greider and Blackburn 1985) and other telomerase-independent mechanisms (Biessman and Mason 1997; Colgin and Reddel 1999), counteracts the progressive shortening of telomeres in highly proliferative cell types such as immortal cell lines, cancer cells, and stem cells. Although telomerase is also expressed in hematopoietic stem cells and lymphocytes, the level of telomerase activity in these cells appears to be insufficient to prevent gradual telomere shortening with age (Hastie et al. 1990; Harley 1995; Rufer et al. 1999).
Although telomere shortening in normal cells is related to the end replication problem (Greider 1996) the mechanisms that result in a variable loss of ∼50–200 bp of telomeric DNA per cell division remain incompletely understood. To address the role of epigenetic factors such as nuclear architecture and chromatin structure in replicative telomere shortening we have employed a novel experimental model based on the study of the X-chromosome telomeres in human female cells. This approach is based on the phenomenon of X-chromosome inactivation (X inactivation) in mammalian cells, where all but one X chromosome are transcriptionally silenced (Lyon 1961). Thus, one of the X chromosomes in normal female mammalian cells becomes facultatively heterochromatic, highly condensed, and tightly associated with the nuclear membrane. The inactive state is initiated early in embryogenesis and then, by epigenetic mechanisms, is maintained in the somatic lineage in all future cell generations (for reviews on X inactivation, see Heard et al. 1997; Willard 1996). The resulting inactive X chromosome (Xi) in mammalian females is distinguished by the lack of acetylated histones, with the practical consequence that antibodies against acetylated histone H4 can be used to visualize the Xi both in metaphase (Jeppesen and Turner 1993; Surrallés and Natarajan 1998a) and in interphase (Surrallés et al. 1996).
In the present study, the length of the telomeres of the X chromosomes and the autosomes was measured in female blood lymphocytes of different ages by combining X-chromosome centromere–specific FISH and quantitative FISH (Q-FISH) with a telomeric peptide nucleic-acid (PNA) probe (Lansdorp et al. 1996; Zijlmans et al. 1997). The activation status of the X chromosomes was simultaneously visualized with antibodies against acetylated histone H4 (see fig 1). By use of this approach, it was possible, for the first time, to measure the age-related telomere shortening of individual chromosomes, including all autosomes, the active X chromosome (Xa), and the Xi.
Material and Methods
Cell Sampling, Culture Procedures, and Acetylated Histone H4 Immunolabeling
Heparinized whole blood was obtained from 15 female donors belonging to three clearly distinguishable age groups with 30–35-year intervals: five samples of umbilical cord blood, five middle-age women (34.6±4.8 years old), and five elderly women (63.4±4.0 years old). Lymphocytes were isolated, cultured, and harvested as described elsewhere (Surrallés et al. 1996). Metaphase spreads from lymphocytes were prepared under acid-free conditions as described elsewhere, to preserve the antigenicity of the histones (Jeppesen and Turner 1993; Surrallés et al. 1996) except that cells were dropped on the slides by use of a Shandon Cytospin-3 cytocentrifuge. After cytocentrifugation, slides were processed for acetylated histone H4 detection, with a rabbit antiserum against acetylated histone H4 and fluorescein isothiocyanate (FITC)–conjugated anti-rabbit antibodies as described in detail elsewhere (Jeppesen and Turner 1993; Surrallés et al. 1996). Then the slides were postfixed in 10% formaldehyde for 15 min at room temperature, rinsed in water, air-dried, and kept until processing by FISH.
FISH with Centromeric and Telomeric PNA Probes
FISH was performed on the slides, with Cy-3–labeled PNA probes for telomeres and X-chromosome centromeres. The details of PNA-FISH methodology are explained elsewhere (Lansdorp et al. 1996; Zijlmans et al. 1997; Martens et al. 1998). Before the processing for PNA-FISH, the slides were pretreated as follows (Surrallés et al. 1996; Surrallés and Natarajan 1998a): 30-s wash in 0.1 M NaOH, 30 s in 10 mM Tris pH 7.5, and 30-s wash in dH20 at room temperature. The slides were then dehydrated with sequential washes in 70%, 90%, and 100% ethanol. The slides were air-dried and washed with PBS, fixed in formaldehyde (4%) in PBS for 2 min, washed again with PBS (three times for 5 min each), and then treated with pepsin at 1 mg/ml at pH 2 (10 min at 37°C). Formaldehyde fixation and washing steps were repeated and slides were dehydrated in increasing series of ethanol and were air-dried. Hybridization mixture containing 70% formamide, 0.5 μg of Cy-3–conjugated telomere (CCCTAA)3/ml and X-chromosome centromere–specific (ATACACTTGCAGATTC and CCCATAACTAAACAC) PNA probes (PBIO/Biosearch Products), and 0.25% (w/v) blocking reagent (DuPont) in 10 mM Tris (pH 7) was placed on the slides and was covered with 24×60-mm coverslips. Slides were denatured for 3 min at 80°C along with hybridization mixture, and hybridization was allowed to occur at room temperature in a humid chamber for 2 h. Slides were then washed with 70% formamide/10 mM Tris (pH 7.2; twice for 15 min each) and with 0.05 M Tris/0.15 M NaCl (pH 7.2) containing 0.05% Tween-20 (three times for 5 min each). After dehydration with an ethanol series, slides were air-dried and counterstained with 0.2 μg of DAPI/ml, in antifading solution Vectashield (Vector Laboratories).
Quantitative Image Analysis
Digital images were recorded with a MicroImager MI1400-12 camera (Xillix) on an Axioplan fluorescence microscope (Zeiss). Microscope control and image acquisition were performed with dedicated software (SSM; Xillix). Separate DAPI and Cy-3 images were subjected to telomere and X-chromosome–centromere fluorescence analysis by use of a dedicated computer program, TFLTELO (Martens et al. 1998). All four telomeres of all chromosomes from a minimum of 15 metaphases per donor were analyzed by Q-FISH. The fluorescence intensities of the Xa and Xi centromeres were measured in exactly the same method and on the same metaphases as were acquired for the telomere length measurements.
Results and Discussion
The telomere fluorescence of both X chromosomes and the autosomes was measured in metaphases from blood lymphocytes of human females of various ages, by combining the PNA probes for X-chromosome centromere–specific FISH and Q-FISH with a PNA telomeric probe. The activation status of the X chromosomes was simultaneously visualized with antibodies against acetylated histone H4 (fig. 1).
Figure 1.
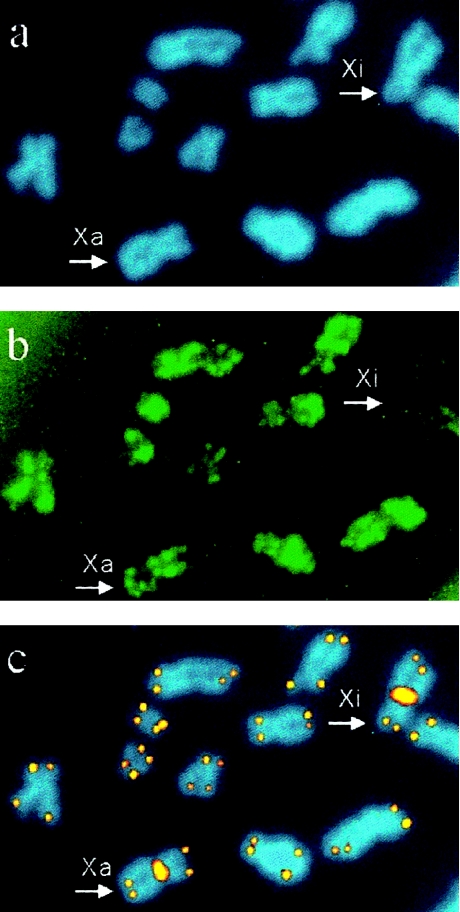
Detection of telomeres of Xa and Xi and of autosomes by simultaneous acetylated histone H4 immunolabeling and FISH with centromeric and telomeric PNA probes. a, Chromosomes counterstained in blue, with DAPI. b, Acetylated histone H4 detected in green FITC fluorescence. c, X-chromosome centromeres and telomeres visualized in orange-yellow Cy-3 fluorescence. Both X chromosomes (arrowhead) are easily distinguishable by the centromeric Cy-3 signal. The Xi is distinguished from the transcriptionally active chromosomes, including the Xa, by its lack of acetylated histone H4 (b, upper right corner).
All four telomeres of each chromosome from a minimum of 15 metaphases per donor were analyzed in the present study, giving a minimum of 900 telomere signals measured in the Xa, 900 in the Xi, and >30,000 in the autosomes. The results are presented in table 1 and are summarized in figure 2. The overall telomere fluorescence decreased with age (P<.001), as expected (Harley 1995). In the middle-aged group, the Xi telomeres were shorter than the telomeres of all other chromosomes, including Xa (P=.002). This difference was even more pronounced in the elderly donors (P<.001) but was not observed in the newborn individuals (P=.8). These results were consistently seen in both short- and long-arm telomeres (table 1). No differences were observed between the Xa telomeres and the telomeres of all chromosomes in any of the age groups or after pooling of the data from the three age groups. When the slopes of the age-related declines obtained with the different chromosomes were compared, the rate of Xi telomere shortening was higher than that observed either for all telomeres on all chromosomes (F=6.4739; P<.01) or for the Xa telomeres (F=7.5372; P<.01). The similar telomere fluorescence in the Xa and Xi chromosomes in the younger age group argues against the differences in the efficiency of telomere FISH between Xi and Xa as an explanation for our findings. This is further supported by the constant centromere fluorescence obtained with a different PNA probe for X-chromosome centromeres in the different age groups (fig. 3). From these results, we conclude that the rate of telomere shortening in Xi is higher than that observed for autosomal or Xa telomeres.
Table 1.
Telomere Fluorescence Intensity in Human Chromosomes as Measured by Q-FISH with a PNA Telomeric Probe
|
Mean
± SE
for |
||||||||||
| All
chromosomes |
Active
X |
Inactive
X |
||||||||
| Donor | Age(years) | All Arms | p Arms | q Arms | All Arms | p Arms | q Arms | All Arms | p Arm | q Arm |
| D1 | 0 | 10.32 ± .2 | 10.45 ± .2 | 10.19 ± .2 | 10.33 ± .5 | 10.35 ± .7 | 10.31 ± .8 | 10.50 ± 1.3 | 10.56 ± 2.4 | 10.44 ± 1.0 |
| D2 | 0 | 9.01 ± .2 | 9.40 ± .3 | 8.63 ± .3 | 9.34 ± .6 | 8.73 ± .7 | 9.96 ± .9 | 8.88 ± .5 | 8.18 ± .7 | 9.59 ± .8 |
| D3 | 0 | 12.70 ± .2 | 13.54 ± .3 | 11.86 ± .3 | 11.79 ± .6 | 12.48 ± .7 | 11.10 ± .9 | 11.48 ± .7 | 12.49 ± 1.1 | 10.48 ± 0.7 |
| D4 | 0 | 11.58 ± .2 | 11.82 ± .3 | 11.34 ± .3 | 11.16 ± .6 | 11.14 ± .8 | 11.18 ± .9 | 10.80 ± .9 | 10.14 ± .7 | 11.47 ± 1.6 |
| D5 | 0 | 10.35 ± .3 | 10.45 ± .4 | 10.25 ± .3 | 9.89 ± .7 | 9.62 ± .9 | 10.16 ± 1.0 | 9.90 ± .6 | 9.96 ± 1.0 | 9.84 ± .8 |
| D6 | 29 | 6.24 ± .1 | 5.59 ± .1 | 6.59 ± .2 | 6.56 ± .4 | 6.62 ± .6 | 6.50 ± .6 | 5.01 ± .2 | 5.31 ± .4 | 4.70 ± .3 |
| D7 | 31 | 5.40 ± .1 | 5.28 ± .1 | 5.52 ± .1 | 5.76 ± .4 | 6.00 ± .5 | 5.51 ± .5 | 4.29 ± .3 | 4.11 ± .4 | 4.48 ± .6 |
| D8 | 34 | 6.44 ± .1 | 6.33 ± .2 | 6.54 ± .2 | 6.88 ± .4 | 7.27 ± .7 | 6.49 ± .5 | 4.05 ± .2 | 4.10 ± .3 | 3.99 ± .3 |
| D9 | 39 | 5.34 ± .1 | 4.79 ± .1 | 6.02 ± .1 | 4.90 ± .3 | 5.17 ± .5 | 4.63 ± .3 | 3.36 ± .1 | 3.53 ± .2 | 3.18 ± .2 |
| D10 | 40 | 5.83 ± .1 | 5.16 ± .1 | 6.57 ± .2 | 5.21 ± .5 | 5.50 ± .6 | 4.92 ± .8 | 4.02 ± .5 | 4.22 ± .8 | 3.82 ± .7 |
| D11 | 60 | 4.72 ± .1 | 4.41 ± .1 | 5.04 ± .1 | 4.27 ± .2 | 4.36 ± .2 | 4.19 ± .3 | 2.25 ± .1 | 2.63 ± .2 | 1.87 ± .1 |
| D12 | 61 | 3.94 ± .1 | 3.99 ± .1 | 3.89 ± .1 | 3.96 ± .3 | 3.88 ± .3 | 4.05 ± .4 | 2.33 ± .2 | 2.71 ± .2 | 1.95 ± .2 |
| D13 | 62 | 3.84 ± .1 | 4.12 ± .1 | 3.55 ± .1 | 3.93 ± .2 | 4.15 ± .2 | 3.71 ± .3 | 2.06 ± .1 | 2.31 ± .2 | 1.80 ± .1 |
| D14 | 64 | 4.31 ± .1 | 3.59 ± .1 | 5.11 ± .1 | 4.50 ± .3 | 4.19 ± .4 | 4.82 ± .4 | 2.43 ± .2 | 2.54 ± .3 | 2.32 ± .2 |
| D15 | 70 | 3.25 ± .1 | 3.39 ± .1 | 3.11 ± .1 | 3.65 ± .2 | 3.35 ± .2 | 3.95 ± .2 | 2.15 ± .1 | 2.58 ± .2 | 1.72 ± .1 |
Figure 2.
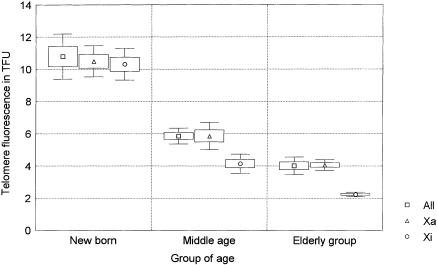
Telomere fluorescence of the Xa, Xi, and all chromosomes (All) measured in lymphocyte metaphases from five newborn, five middle-aged (34.6±4.8 years old), and five elderly females (63.4±4.0 years old). Fluorescence intensities of all four telomeres of all the chromosomes from a minimum of 15 metaphases per donor were measured by Q-FISH with a telomeric PNA probe. Mean fluorescence intensities, standard errors, and SD are represented by middle points (squares, triangles, and circles), boxes, and whiskers, respectively.
Figure 3.
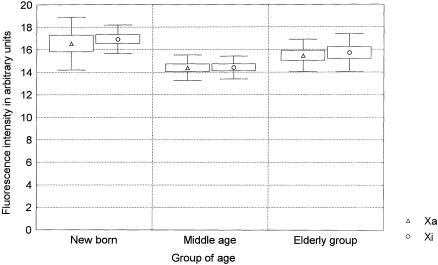
Centromere fluorescence of the Xa and Xi measured in the same lymphocyte metaphases acquired to generate figure 2. The centromere intensity of the Xa and the Xi was measured by Q-FISH with a centromeric PNA probe. Mean fluorescence intensities, standard errors, and SD are represented by middle points (triangles and circles), boxes and whiskers, respectively.
To our knowledge, this is the first evidence to show a differential rate of telomere shortening between and within homologous chromosomes in any living organisms. Considering that Xi telomeres are part of the highly condensed Barr body, our results could be explained by a lower accessibility of Xi telomeres to telomere-maintenance mechanisms such as telomerase or recombination. This would be consistent with the laser-scanning confocal-microscopy observation that the Xi telomeres are internally located in the Xi nuclear region (Dietzel el al. 1998). According to this idea, the rate of telomere shortening in the Xa and Xi would be similar until a critical length is reached. Recent studies suggest that all mammalian telomeres end in a loop structure (Griffith et al. 1999). The minimum length to form such a loop or the consequences of reaching the threshold telomere length could be different in the Xa and Xi. The involvement of DNA-repair pathways in normal telomere maintenance (d'Adda di Fagagna et al. 1999) is compatible with the observation that short telomeres appear to accumulate prior to replicative senescence in cultures of diploid human fibroblasts (U. M. Martens, E. A. Chavez, S. S. S. Poon, C. Schmoor, P. M. Lasdorp, personal communication). Failure of short telomeres to trigger an efficient DNA-damage response or inefficient DNA repair at telomeres of the Xi would result in continued telomere shortening in the Xi. This is consistent with the reported heterogeneity in X-ray–induced double-strand break repair between the Xa and Xi (Surrallés and Natarajan 1998b).
In mouse (Zijlmans et al. 1997) and hamster (Slijepcevic et al. 1997) chromosomes, telomeres close to the centromere (p-arm telomeres) are shorter than telomeres more distant to centromeres (q-arm telomeres), suggesting that centromere position might exert some distance-dependent effect on telomere length (Slijepcevic 1998). This could also explain our observations, considering that the condensed structure of the Barr body could lead to a shorter distance between telomeres and centromeres in the Xi. Alternatively, the late replication of the Xi (Willard and Latt 1976) could decrease telomere extension by recombination or by telomerase. Possibly, X inactivation itself could lead to accelerated telomere shortening. Whatever the explanation, our results suggest that the rate of replicative telomere shortening in aging human cells is not constant but is modulated by epigenetic factors.
Telomeres are known to play a key role in chromosome segregation (Sandell and Zakian 1993; Blasco et al. 1997; Kirk et al. 1997; Hande et al. 1999). Since the pioneering work of Jacobs and coworkers in the early 1960s, it has been known that the rate of aneuploidy in cultured lymphocytes increases with advancing age (Jacobs et al. 1961, 1963), with an extreme overinvolvement of the X chromosome in women (Fitzgerald and McEwan 1977). Perhaps the loss of a sex chromosome is tolerated better than the loss of an autosomal chromosome. Although selective survival may explain the high frequency of cells with sex-chromosome aneuploidy, the mechanism by which such chromosomes are so frequently lost is not known. According to our results, an age-related loss of centromeric alphoid sequences of the X chromosomes is unlikely, since the centromeric fluorescence of both X chromosomes is stable with increasing age (fig. 3). However, our data are consistent with a potential causative role of telomere shortening in the age-related sex-chromosome aneuploidy in humans. Thus, although the decrease in telomere length is more substantial at a young age than in later years (Rufer et al. 1999; present study), telomeres would reach a critical short length and lose their function in chromosome segregation in older individuals, especially in the Xi. Specifically designed studies are needed to further test this hypothesis.
Acknowledgments
Special thanks are due to the José Carreras Foundation Cord Blood Program, Dr. Sergi Querol (Spanish Eurocord Cord Blood Bank, Cancer Research Institute, Barcelona), and Drs. Francesc Baró and Albert Cabero (Gynaecology and Obstetrics Service, Hospital Vall d'Hebron, Barcelona) for giving us the blood samples required to perform this study. We thank María José Ramírez for her help in the statistical analysis. We also thank Dr. Bryan M. Turner (Anatomy Department, Universityof Birmingham Medical School, UK) for the gift of the antiacetylated histone H4 rabbit antiserum. This work was partially funded by Spanish Ministry of Education and Culture grant PM98-0179, the Commission of the European Union, and Fondo de Investigación Sanitaria, Spanish Ministry of Health grant FIS 99/1214. Work by M.P.H. and P.M.L. in this study was supported by a grant from the National Cancer Institute of Canada, with funds from Terry Fox Run. J.S. is supported by a Contrato de Incorporación de Doctores awarded by the Spanish Ministry of Education and Culture.
Footnotes
* The first two authors contributed equally to this work.
References
- Biessman H, Mason JM (1997) Telomere maintenance without telomerase. Chromosoma 106:63–69 [DOI] [PubMed]
- Blasco MA, Lee H-W, Hande P, Samper E, Lansdorp P, DePinho R, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25–34 [DOI] [PubMed]
- Colgin LM, Reddel RR (1999) Telomere maintenance mechanisms and cellular immortalization. Curr Opin Genet Dev 9:97–103 [DOI] [PubMed]
- d'Adda di Fagagna F, Hande MP, Tong W-M, Lansdorp PM, Wang Z-Q, Jackson SP (1999) Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nat Genet 23:76–80 [DOI] [PubMed]
- Dietzel S, Eils R, Sätzler K, Bornfleth H, Jauch A, Cremer C, Cremer T (1998) Evidence agaist a looped structure of the inactive human X-chromosome territory. Exp Cell Res 240:187–196 [DOI] [PubMed]
- Fitzgerald PH, McEwan CM (1977) Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet 39:329–337 [DOI] [PubMed]
- Greider CW (1996) Telomere length regulation. Annu Rev Biochem 65:337–365 [DOI] [PubMed]
- Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413 [DOI] [PubMed]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97:503–514 [DOI] [PubMed]
- Hande MP, Samper E, Lansdorp P, Blasco MA (1999) Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J Cell Biol 144:589–601 [DOI] [PMC free article] [PubMed]
- Harley CB (1995) Telomeres and aging. In: Blackburn EH, Greider CW (eds) Telomeres, Cold Spring Harbor, NY: Cold Spring Harbor Press, pp 247–263 [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC (1990) Telomere reduction in human colorectal carcinoma and with ageing. Nature 346:866–868 [DOI] [PubMed]
- Heard E, Clerc P, Avner P (1997) X-chromosome inactivation in mammals. Annu Rev Genet 31:571–610 [DOI] [PubMed]
- Jacobs PA, Brunton M, Court Brown WM, Doll R (1963) Change in human chromosome count with age: evidence for a sex difference. Nature 197:1080–1081 [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Court Brown WM, Doll R (1961) Distribution of human chromosome counts in relation to age. Nature 191:1178–1180 [DOI] [PubMed] [Google Scholar]
- Jeppesen P, Turner BM (1993) The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell 74:281–289 [DOI] [PubMed]
- Kirk KE, Harmon BP, Reichardt IK, Sedat JW, Blackburn EH (1997) Block in anaphase chromosome separation caused by telomerase template mutation. Science 275:1478–1481 [DOI] [PubMed]
- Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little M-T, Dirks RW, Raap AK, et al (1996) Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 5:685–691 [DOI] [PubMed]
- Lyon MF (1961) Gene action in the X chromosome of the mouse (Mus musculus). Nature 190:372–373 [DOI] [PubMed] [Google Scholar]
- Martens UMM, Zijlmans MJP, Poon SSS, Dragowska W, Yui J, Chavez EA, Ward RK, et al (1998) Short telomeres on human chromosome 17p. Nat Genet 18:76–80 [DOI] [PubMed]
- Rufer N, Brümmendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, et al (1999) Telomere fluorescence measurements in granulocytes and T lymphocytes subsets points to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190:157–167 [DOI] [PMC free article] [PubMed]
- Sandell LL, Zakian VA (1993) Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell 75:729–739 [DOI] [PubMed]
- Slijepcevic P (1998) Telomere length regulation: a view from the individual chromosome perspective. Exp Cell Res 244:268–274 [DOI] [PubMed]
- Slijepcevic P, Hande MP, Bouffler SD, Lansdorp PM (1997) Telomere length, chromatin structure and chromosome fusigenic potential. Chromosoma 106:413–421 [DOI] [PubMed]
- Surrallés J, Jeppesen P, Morrison H, Natarajan AT (1996) Analysis of loss of inactive X chromosome in interphase cells. Am J Hum Genet 59:1091–1096 [PMC free article] [PubMed]
- Surrallés J, Natarajan AT (1998a) Position effect of translocations involving the inactive X chromosome: physical linkage to XIC/XIST does not lead to de novo inactivation in human differentiated cells. Cytogenet Cell Genet 82:58–66 [DOI] [PubMed]
- ———(1998b) Radiosensitivity and repair of the inactive X-chromosome: insights from FISH and immunocytogenetics. Mutat Res 414:117–124 [DOI] [PubMed]
- Willard HF (1996) X-chromosome inactivation, XIST, and pursuit of the X-inactivation center. Cell 86:5–7 [DOI] [PubMed]
- Willard HF, Latt SA (1976) Analysis of deoxyribonucleic acid replication in human X chromosomes by fluorescence microscopy. Am J Hum Genet 28:213–227 [PMC free article] [PubMed]
- Zijlmans JM, Martens UM, Poon S, Raap AAK, Tanke HJ, Ward RK, Lansdorp PM (1997) Telomeres in the mouse have large inter-chromosomal variation in the number of T2AG3 repeats. Proc Natl Acad Sci USA 94:7423–7428 [DOI] [PMC free article] [PubMed]


