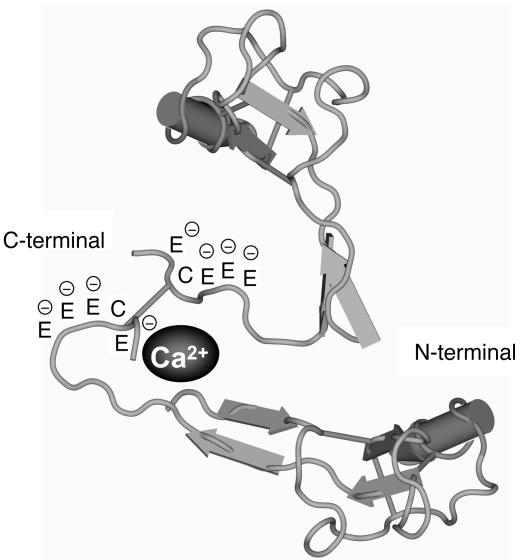
Figure 10.
The proposed model of Ca2+-binding site in the TFF1 molecules. Based on the functional data in Figure 7D, and because the C terminus contains multiple glutamic residues that are negatively charged, we therefore hypothesize that this area is particularly important in mediating the CaOx crystal growth–inhibitory function of TFF1. Because dimerization usually occurs in the native form of TFF1 (Cys58–Cys58) (30), we also propose that dimerization of TFF1 may facilitate entrapment of Ca2+ ions in this area.