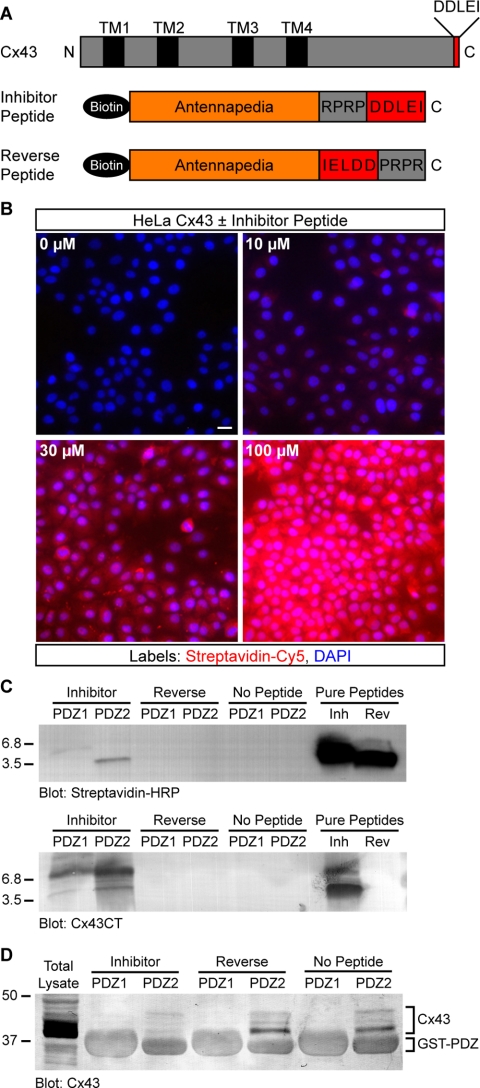
Figure 4.
A peptide inhibitor that binds specifically to the second PDZ domain of ZO-1 and blocks interaction with Cx43. (A) Domain organization of full-length Cx43 and antennapedia peptides. (B) Detection of biotinylated peptide (0–100 μM) in HeLa cells after 2 h. Streptavidin-Cy5 fluorescence intensity shows a dose-dependent increase with increasing peptide concentration. Cell nuclei are stained with DAPI. Bar, 15 μm. (C) Western blots showing that inhibitor and reverse peptides (∼3.5 kDa) both were detected by streptavidin-HRP (top blot), but only inhibitor peptide was detected by an antibody that recognizes the C terminus of Cx43 (bottom blot). Only inhibitor peptide was pulled down by purified ZO-1 GST-PDZ2 fusion protein-coupled beads; neither peptide was pulled down by GST-PDZ1 beads. (D) Representative Cx43 blot showing that the amount of endogenous Cx43 pulled down from HeLa lysates by GST-PDZ2 beads was significantly reduced by the presence of inhibitor peptide. Peptide inhibition of PDZ2-Cx43 interaction was reproducible (n = 3). Comparison of nonspecific GST fusion protein band intensities confirms equal loading across conditions.