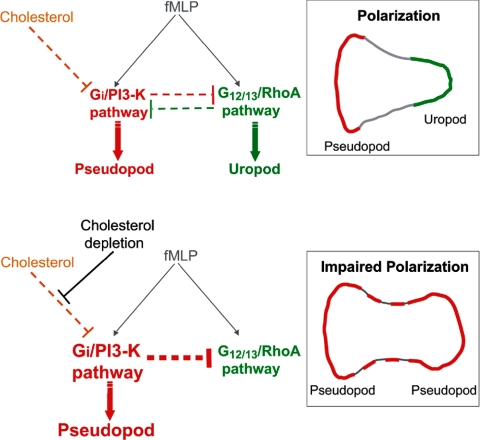
Figure 7.
A model for the role of cholesterol in membrane organization during neutrophil polarization and migration. During polarization and migration (top diagram), activation of the fMLP-receptor at the front stimulates the Gi/PI3-K pathway (red), which promotes actin polymerization and protrusion. Receptor activation at the rear stimulates the G12/13/RhoA pathway (green), which promotes actin-myosin contraction. These pathways segregate and are maintained at opposite poles of the cell in part via mutual negative feedback pathways (dotted red and green lines; Xu et al., 2003). Cholesterol is critical for the formation of membrane domains that enable the spatial segregation of signaling pathways to the front and rear of cells. Cholesterol may directly influence the activation of signaling proteins involved in the Gi/PI3-K pathway and inhibit lateral diffusion of D3-PIs. Cholesterol may also down-regulate the Gi/PI3-K pathway in the uropod, inhibiting negative feedback on the RhoA pathway and promoting uropod formation. In response to cholesterol depletion by MβCD (bottom diagram), activation of the Gi/PI3-K pathway (red) is enhanced, leading to D3-PI accumulation over the entire plasma membrane, and to actin polymerization and the formation of multiple protrusions. This in turn enhances negative feedback on the RhoA pathway (green), inhibiting uropod function.