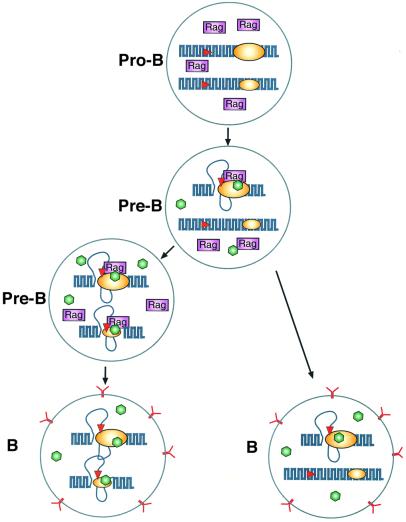
Fig. 5. A model for pre-programmed allelic exclusion. The diagram shows the κ alleles in B cells at different stages of lymphoid development. In pro-B cells, both alleles are in a closed chromatin conformation, and for this reason the Rag proteins present in these cells have no access to the RSS sites (red) of the locus. In pre-B cells, specific factors (green) can activate the Igκ enhancer system (yellow) and open local chromatin. The early replicating allele represents a better target (large yellow ellipse) than the late replicating allele (small yellow ellipse). Once opened, the RSS serves as a substrate for the Rag proteins (purple) that bring about rearrangement. If this results in the generation of a productive cell surface antigen receptor (Y), Rag expression is then inhibited in B cells (right). In the event that the first rearrangement is not productive, with time, the second allele can become accessible and undergo recombination (left), which may again lead to an antibody-producing cell (bottom). It should be noted that in cells containing a productive rearrangement of the first allele (right), the trans-acting enhancer factors (green) are presumably still present, and this could lead to opening of the second allele. Since Rag proteins are not present, however, these will not undergo rearrangement. It is for this reason that one observes unmethylated germ line alleles in a small percentage of mature B cells. The fact that most B cells have only a single accessible κ allele also explains why secondary changes resulting from editing or somatic hypermutation appear to take place preferentially on alleles that have already undergone at least one round of rearrangement.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.