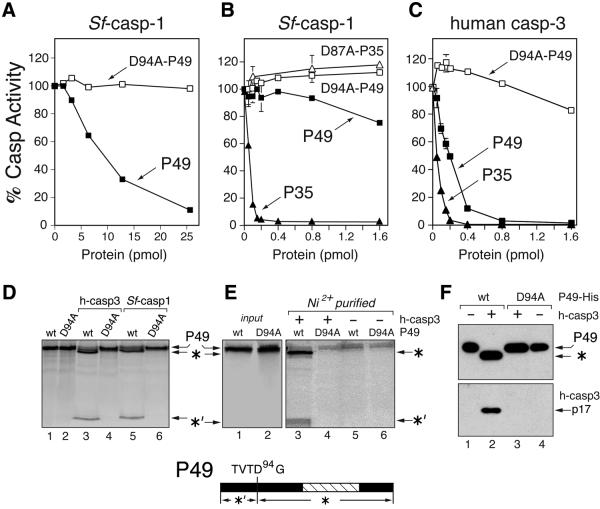
Fig. 2. P49 is a substrate inhibitor. (A and B) Inhibition of Sf-caspase-1. Purified recombinant Sf-caspase-1 (0.1 pmol) was incubated with the indicated amounts of purified P49-His6 (filled square), D94A-mutated P49-His6 (open square), P35-His6 (filled triangle) or D87A-mutated P35-His6 (open triangle). After 30 min, residual caspase activity was measured by using DEVD-amc as substrate. Plotted values are the average ± standard deviation of triplicate assays and are expressed as a percentage of uninhibited caspase activity. (A) and (B) differ in the range of P49 used. (C) Inhibition of human caspase-3. Purified recombinant caspase-3 (0.1 pmol) was incubated with the indicated amounts of purified P49-His6 (filled square), D94A- mutated P49-His6 (open square) or P35-His6 (filled triangle) and assayed as described above. (D) P49 cleavage. In vitro synthesized, 35S-radiolabeled, wild-type (wt) or D94A-mutated P49 was mixed with buffer (lanes 1 and 2), human caspase-3 (lanes 3 and 4) or Sf-caspase-1 (lanes 5 and 6) and analyzed by SDS–PAGE. The 40 kDa (*) and 9 kDa (*′) cleavage fragments are indicated (bottom). (E) P49–caspase-His6 complexes. Radiolabeled untagged wild-type or D94A-mutated P49 was mixed with buffer or His6-tagged human caspase-3 (300 pmol). Complexes recovered by Ni2+ affinity chromatography in the presence (lanes 3 and 4) or absence (lanes 5 and 6) of caspase-3 were analyzed by SDS–PAGE. Uncleaved P49 (input) was included (lanes 1 and 2). (F) P49-His6–caspase complexes. Purified wild-type (wt) or D94A-mutated P49-His6 was mixed with buffer or untagged caspase-3. Recovered Ni2+-bound complexes were subjected to immunoblot analysis by using α-P49 (top) or caspase-3 large subunit α-P17 (bottom) antisera.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.