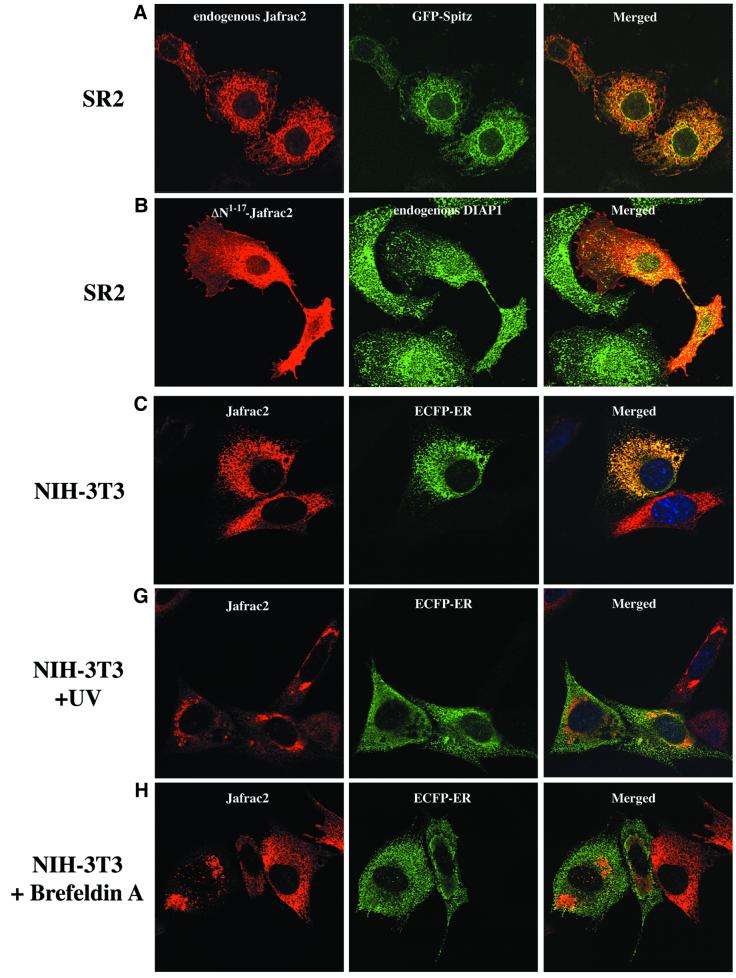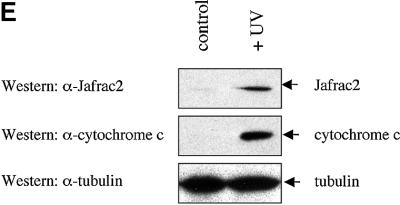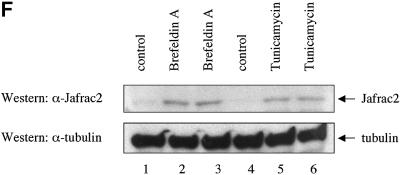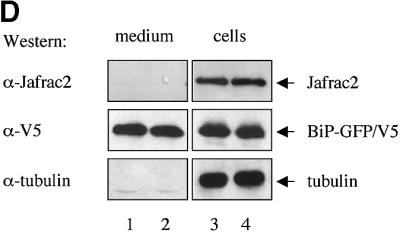
Fig. 2. Subcellular localization of Jafrac2. (A) Endogenous Jafrac2 resides in the ER. Immunofluorescence confocal microscopy of SR2 cells stained with an anti-Jafrac2 antibody (red) and GFP–Spitz (green). Right panels represent merged micrographs. (B) The N-terminal 17 Aa of Jafrac2 are required for targeting Jafrac2 to the ER. Confocal micrographs of SR2 cells transfected with ΔN1–17-Jafrac2 lacking the N-terminal signal sequence and stained with anti-V5 (red) and anti-DIAP1 antibodies (green). (C) Confocal micrographs of NIH 3T3 cells transfected with Jafrac2 (red) and the ER-marker ECFP-ER (green). (D) Jafrac2 is an intracellular protein. Endogenous Jafrac2 is present exclusively in the cellular but absent from the medium fraction. (E) Jafrac2 is released from the ER in UV-mediated apoptosis. Normal and UV-treated S2 cells were lysed in a buffer containing 0.025% digitonin and subfractionated into membrane and cytosolic fractions. The cytosolic fractions were examined by immunoblot analysis with the indicated antibodies. (F) Jafrac2 is released from the ER by the ER stress-inducing agents brefeldin A or tunicamycin. Normal and treated NIH 3T3 cells stably expressing Jafrac2-V5 were analysed as in (E). At 3–4 h post-UV (G) or -brefeldin A treatment (H) of NIH 3T3, the majority of Jafrac2 (red) is no longer co-localized with ECFP-ER (green), as seen by overlaying the green and red images (yellow).