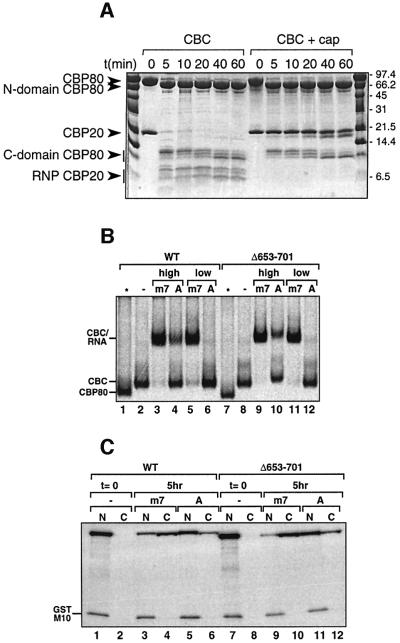
Fig. 1. (A) Time course of limited proteolysis. A 42 µg aliquot of CBC was incubated at room temperature with 210 ng of trypsin in the absence or presence of 10 mM cap analogue m7GpppG in a total volume of 60 µl. Aliquots of 10 µl were taken off every 0, 5, 10, 20, 40 and 60 min, denatured by 3 µl of denaturing buffer (125 mM Tris pH 6.8, 260 mM DTT, 30% glycerol, 10% SDS and 0.025% Coomassie Blue) and loaded onto a 13.5% Tricine SDS–polyacrylamide gel. (B) Cap-binding activity of CBP80 Δ653–701. [35S]Methionine-labelled wild-type or mutant CBP80 was incubated for 30 min at room temperature in the absence (–) or presence of either 1.7 µM (high) or 53 nM (low) m7GpppG-capped (m7) or ApppG-capped (A) unlabelled U1ΔSm RNAs. The samples were fractionated by native 6% PAGE followed by fluorography. Free CBP80, CBC and the CBC–RNA complex are indicated. In the lanes indicated by an asterisk, the corresponding CBP80 proteins were loaded without CBP20. (C) Shuttling activity of CBP80ΔNLSΔ653–701 in Xenopus oocytes. [35S]methionine-labelled CBP80ΔNLS2 or CBP80ΔNLS2Δ653–701 were injected together with [35S]methionine-labelled GST–M10 into Xenopus oocyte nuclei either (1) alone (lanes 1 and 2, and 7 and 8), (2) together with m7GpppG-capped unlabelled U1ΔSm RNAs (lanes 3 and 4, and 9 and 10) or (3) together with ApppG-capped U1ΔSm RNAs (lanes 5 and 6, and 11 and12). Oocytes were dissected either immediately (lanes 1 and 2, and 7 and 8) or 5 h after injection (lanes 3–6 and 9–12) and the proteins analysed by SDS–PAGE followed by fluorography. GST–M10 is a mutant of HIV Rev with a non-functional nuclear export signal used as a negative control. See Ohno et al. (2000) or Segref et al. (2001) for more details.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.