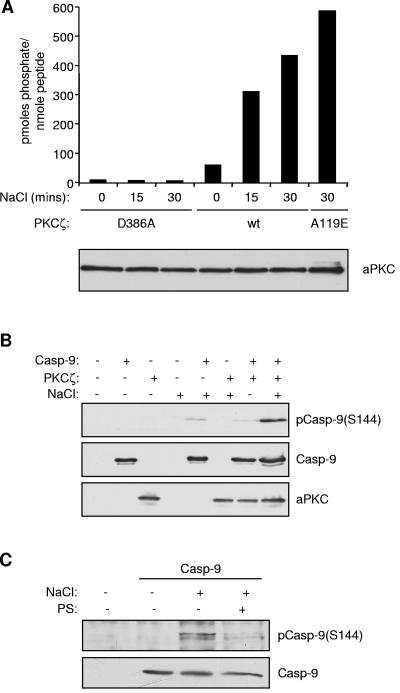
FIG. 9.
Hyperosmotic stress induces PKCζ activation and caspase 9 (Casp-9) phosphorylation at Ser144. (A) Hyperosmotic stress increases PKCζ activity. HEK293 cells were transfected with wild-type (wt), constitutively active (A119E), or kinase-dead (D386A), FLAG-tagged PKCζ. At 24 h posttransfection, cells were serum starved for a further 24 h and then treated with 0.7 M NaCl for the times shown. FLAG-PKCζ was immunoprecipitated from cell lysates and assayed for kinase activity. The amount of aPKC (ζ/λ) recovered in each sample and detected by Western blotting is shown below. (B) HEK293 cells were cotransfected with caspase 9 (C287A) and PKCζ or empty vector. Cells were treated with 0.7 M NaCl for 30 min, and then cell lysates were analyzed by immunoblotting with phospho-Ser144, caspase 9, and aPKC antibodies, as indicated. (C) Phosphorylation of endogenous caspase 9 on Ser144 in response to osmotic stress. HeLa cells were serum starved for 48 h and then treated with 100 μM pseudosubstrate inhibitor (PS) for 45 min, followed by 0.7 M NaCl for 45 min. Caspase 9 immunoprecipitates were Western blotted as in panel A.