FIG. 1.
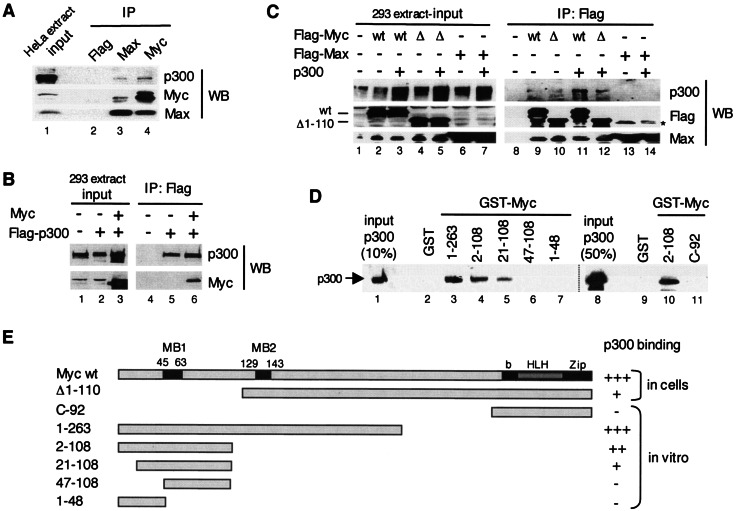
Myc interacts with p300 in mammalian cells and in vitro via its TAD. (A) A HeLa whole-cell extract was immunoprecipitated (IP) with Flag (lane 2), Max H2 (lane 3), and Myc C-33 (lane 4) antibodies and analyzed by Western blotting (WB). Different stripes of the same blot were probed with p300 (N-15), Myc (N-262), and Max (C-17) antibodies. Lane 1 shows the input extract. (B) Extracts of HEK293 cells transfected with pUHD-Myc and/or pCI-p300-Flag, or the corresponding empty vectors as indicated with “+” and “−,” respectively (input, lanes 1 to 3), were immunoprecipitated with the Flag antibody (IP: Flag, lanes 4 to 6) and analyzed by Western blotting (WB) with p300 (N-15) and Myc (N-262) antibodies. (C) Extracts of HEK293 cells (input, lanes 1 to 7) transfected with Flag-Myc wild-type (wt), Flag-Myc deletion mutant Δ1-110 (Δ), Flag-Max, and p300, or corresponding empty vectors as indicated (+ and −), were immunoprecipitated with the Flag antibody (IP: Flag, lanes 8 to 14) and analyzed by Western blotting with p300, Flag, and Max antibodies, as indicated (WB). The positions of Myc wt and Δ1-110 are indicated. A nonspecific band was detected in lanes 13 and 14 (*). (D) GST pull-down assays. Purified recombinant p300 (lanes 1 and 8; see also Fig. 2A) was incubated with immobilized GST (lanes 2 and 9) and GST-Myc fusion proteins with different Myc regions as indicated (lanes 3 to 7, 10, and 11, see also panel E). Specific binding of p300 was analyzed by Western blotting with p300 (N-15) antibodies. (E) Summary of the above p300-Myc interaction results obtained by coimmunoprecipitation assays (in cells) and by GST pull-down assays (in vitro). MB1 and MB2 are Myc boxes 1 and 2; bHLHZip is the basic helix-loop-helix leucine zipper domain. Strong interaction with p300, i.e., comparable to wild-type Myc (+++), medium (++), weak (+), and no detectable (−) interactions are indicated.