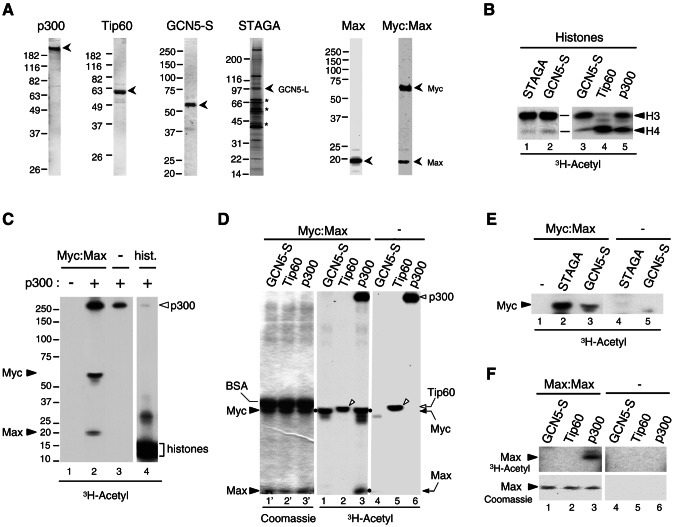
FIG. 2.
Acetylation of the Myc-Max complex by p300, GCN5, and STAGA but not Tip60 in vitro. (A) Purified recombinant proteins analyzed by SDS-PAGE and stained with Coomassie blue: p300 (1 μg), Tip60α (1 μg), GCN5-S (0.2 μg), Max (1 μg), and Myc-Max heterodimer (1 pmol). The STAGA complex (8 μl) was stained with silver, and asterisks indicate nonspecific proteins. Positions of molecular mass standards are on the left. (B) In vitro acetylation of histones with the indicated HATs and [3H]acetyl-CoA was analyzed by SDS-PAGE and fluorography (3H-Acetyl). Acetylated histones H3 and H4 are indicated. (C) In vitro acetylation of the Myc-Max complex (2 pmol, lanes 1 and 2) and histones (25 pmol, lane 4) without (−) and with (+) 4 ng of purified recombinant p300 as indicated, was analyzed by SDS-PAGE and fluorography. Lanes 1 to 3 and lane4 are from distinct regions of the same gel (spliced together) and same autoradiographic exposure (33 h). Acetylated Myc, Max, p300, and histones are indicated. (D) In vitro acetylation (as described above) with Myc-Max complex and the indicated HATs normalized for their HAT activity (see panel B). Myc-Max was omitted in lanes 4 to 6. The left panel (lanes 1′ to 3′) is the Coomassie blue-stained gel of the middle fluorographic image (lanes 1 to 3). Coomassie blue-stained BSA, Myc and Max are indicated on the left. The positions of acetylated Myc and Max are indicated on the right and with dots next to the respective bands in lanes 1 and 3. Autoacetylated p300 and Tip60 are indicated on the right (open arrowhead and arrow, respectively). Autoacetylated Tip60 is indicated with open arrowheads in lanes 2 and 5. (E) In vitro acetylation of the Myc-Max complex, as described above, but with normalized GCN5-S and STAGA. (F) In vitro acetylation of Max-Max homodimer (lanes 1 to 3), as in panel D. Max-Max was omitted in lanes 4 to 6. The top panels show an autoradiography of the portion of the Coomassie blue-stained gel shown in the bottom panels.