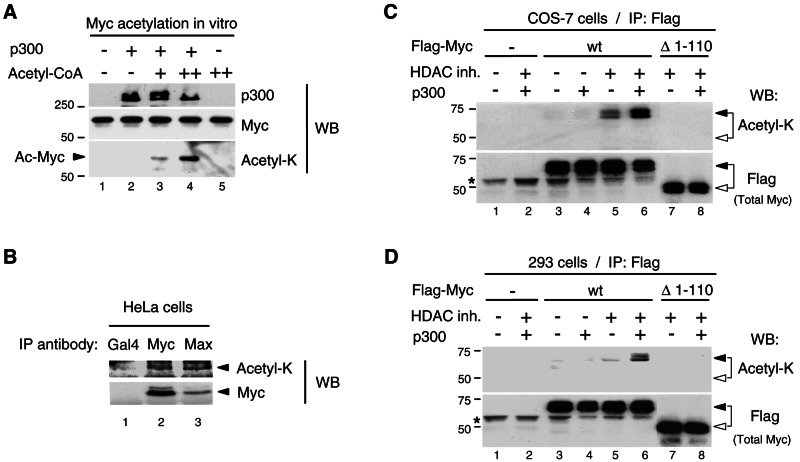
FIG. 3.
Myc is acetylated by p300 in mammalian cells. (A) Acetyl-K antibody recognizes p300-acetylated Myc in vitro. The Myc:Max complex (0.3 pmol) was acetylated in vitro in the absence (−) and presence (+) of p300 (50 ng) and cold acetyl-CoA (2 μM, lane 3, and 10 μM, lanes 4 and 5). The reactions were analyzed by Western blotting (WB) with p300 (N-15), Myc (C-33), and acetyl-K antibodies. The Myc portion of the filter was probed first with acetyl-K antibody and then stripped and reprobed with Myc (C-33) antibody. Acetylated Myc is indicated with an arrowhead. (B) Endogenous Myc-Max complexes were immunoprecipitated with Myc (N-262) and Max (C-124) antibodies (lanes 2 and 3, respectively) from HeLa cells transfected with p300 and treated for 4.5 h with HDAC inhibitors and MG-132. A control immunoprecipitation was performed with a Gal4 (DBD) antibody (lane 1). Western blot was performed first with the acetyl-K antibody and, after stripping the same filter, was reprobed with Myc (C-33) antibody. TrueBlot reagents were used for IP and Western blotting (see Materials and Methods). Arrowheads indicate the position of Myc. (C) COS-7 cells and (D) HEK293 cells were transfected as described in Materials and Methods with either Flag-Myc wt or Flag-MycΔ1-110 mutant (Δ1-110) and p300 (+) or the corresponding empty vectors (−), as indicated. Cells were treated (+) or mock-treated (−) with HDAC inhibitors (HDAC inh.), as indicated. Immunoprecipitations were performed with the Flag antibody and Flag-Myc proteins were analyzed by Western blotting (WB) with the acetyl-K antibody first (top panel) and then, after stripping the filter, with the Flag antibody (bottom panel). Flag-Myc wt and Δ1-110 are indicated with filled and open arrowheads, respectively. An asterisk indicates the immunoglobulin H chain.