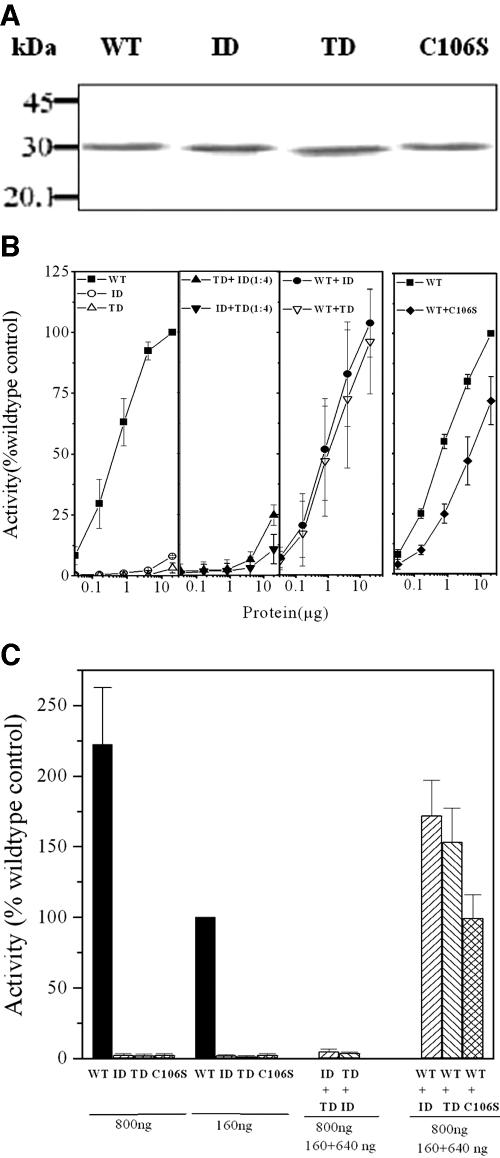
FIG. 2.
Assessment of deformylase activities of mPDF mutants. (A) Western blotting of WT and mutant (ID, TD, and C106S) proteins using anti-His-tag antibody. (B) Deformylase activities of mPDF mutants. WT and mutant proteins were mixed with each other (ID+TD, TD+ID, WT+ID, WT+TD, or WT+C106S) at the ratio of 1:4 after denaturation with urea. They were refolded and subsequently purified as mentioned above. Enzyme activities were monitored with increasing concentrations of total proteins in the presence of catalase and BSA by using 5 mM of N-formyl-Met-Ala as the substrate in the TNBSA assay (17). (Left, panels 1 to 3) Assays with WT, ID, TD, ID+TD, TD+ID, WT+ID, and WT+TD proteins. (Right) Assays with WT and WT+C106S proteins. Results are expressed as percentages of enzyme activity obtained with 20 μg WT protein. (C) Enzyme activities with cofolded proteins. Assays were carried out with indicated amount of proteins (WT, ID, TD, ID+TD, TD+ID, WT+ID, WT+TD, or WT+C106S) in the presence of catalase and BSA by using 5 mM of N-formyl-Met-Ala as the substrate (17). Results are expressed as the percentages of enzyme activity obtained with 160 ng WT protein (100% = 3.24 ± 0.7 μmol free amino group produced/min/mg protein).