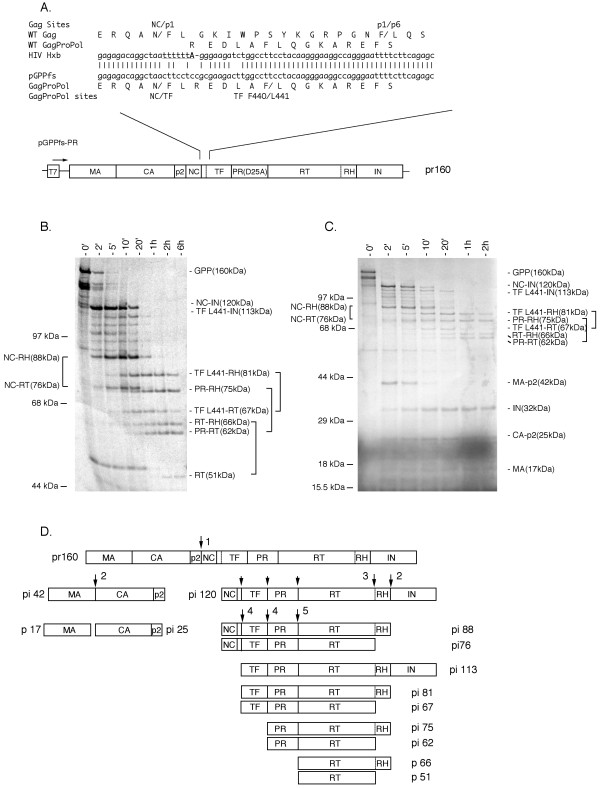
Figure 1.
A. The frameshift mutation in pGPPfs-PR. Above: the sequence of wild type HIV-1 HXB (GenBank:NC001802) molecular clone in the area of translational frameshift in gag-pro-pol is shown. The heptanucleotide slippery sequence required for translational frameshifting is underlined [23, 24]. The adenine that is read twice during frameshifting is shown in bold. The exact site of frameshifting in the wild type virus is variable with 70% of Gag-Pro-Pol product containing Leu as the second residue of the transframe domain (TF) [27]. pGPPfs-PR expressed in vitro in a coupled transcription/translation system [28] gives the predominant Gag-Pro-Pol product. Additional translationally silent substitutions were inserted in the area frameshift to reduce secondary structure and translational pausing during expression. The activity of the intrinsic protease was inactivated by a D25A substitution of the catalytic aspartate. The location of the Gag NC/p1 [53] and pl/p6 [54] sites and the Gag-Pro-Pol NC/TF and TF F440/L441 sites [28, 32, 33, 35] are also shown. Below: an overall schematic pGPPfs-PR. B, C. Processing of the HIV-1 Gag-Pro-Pol precursor in vitro showing the kinetics of processing and the generation of product pairs over time. The full-length Gag-Pro-Pol pr160 precursor containing an inactive protease (by PR D25A mutation of the catalytic aspartate) was generated by transcription and translation of pGPPfs-PR in a rabbit reticulocyte lysate. Purified mature HIV-1 protease was added in trans following the 0' timepoint. Aliquots were removed at the indicated time and the protein products separated by Tris-Glycine SDS-PAGE (B) [30] or by Tris-Tricine SDS-PAGE (C) [31]. Paired products resulting from prior removal of IN followed by partial cleavage at the RT/RH site are denoted with brackets. Molecular mass markers are shown on the left. The molecular masses of the intermediates and final products, as estimated from published sequence or common nomenclature, are also shown. Products are represented in abbreviated form by the N- and C-terminal domains according to the nomenclature of Leis et al. [55]. D. Proposed pathway for the ordered processing of the HIV-1 Gag-Pro-Pol precursor by protease in trans. The Gag-Pro-Pol precursor and the observed predominant processing intermediates are represented as boxes with processing sites denoted as vertical lines. The schematic separates the observed Gag-Pro-Pol cleavages into distinct rates. The initial cleavage at p2/NC is shown with a large arrow and labeled 1. The next cleavages occur with similar rates and are labeled 2 (RH/IN and MA/CA). This cleavage is quickly followed by half-cleavage at the RT/RH site (labeled 3). A series of intermediates between 120 kDa and 88 kDa are accounted for at least in part by early cleavage at the sites upstream of RT (TF F440/L441, TF/PR, PR/RT), and these are indicated with small arrows. The slower cleavages at these sites (labeled 4 and 5) give rise to the later paired products. The molecular masses shown of the intermediates and final products were estimated from published sequence or common nomenclature.