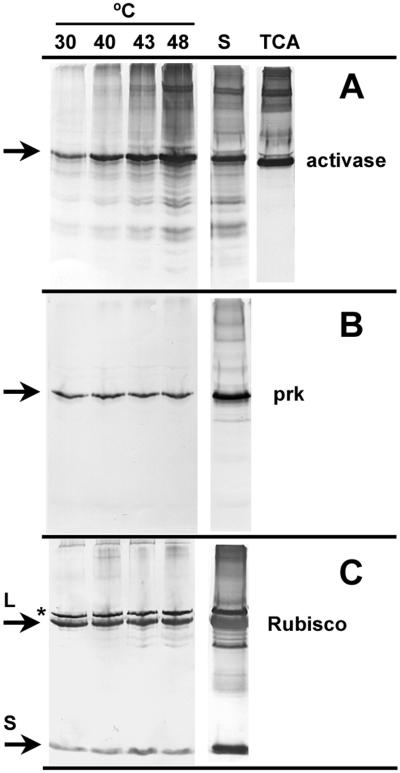
Figure 7.
Effect of temperature on thermal aggregation of activase, phosphoribulokinase, and Rubisco from heat-treated tobacco protoplasts. Intact protoplasts were incubated for 10 min at the indicated temperatures and then lysed with detergent in the presence of rhodanese. After 5 min at 23°C, aggregated protein was collected by centrifugation and separated by SDS-PAGE. Proteins in the soluble portion of the extract from the 48°C incubation (S) and in protoplasts that were incubated at 48°C and then lysed directly in TCA were also separated by SDS-PAGE after diluting 3-fold. Individual polypeptides were visualized by immunoblotting using antibodies to activase (A), phosphoribulokinase (prk, B), and Rubisco (C). The arrows indicate the position of activase (42 kD), phosphoribulokinase (43 kD), and the large (L, 53 kD) and small (S, 14 kD) subunits of Rubisco on the blots. The polypeptide labeled with an asterisk is an unidentified chloroplast protein that cross-reacts with Rubisco antibodies.