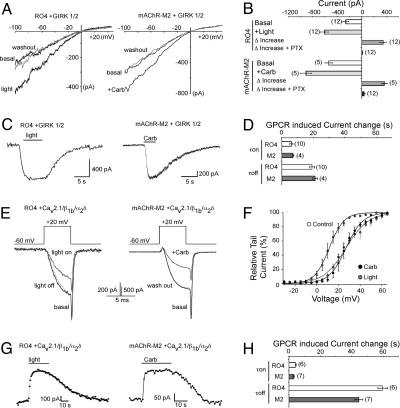
Fig. 1.
Vertebrate rhodopsin modulates GIRK and P/Q-type Ca2+ channels via Gi/o-PTX-sensitive pathways. (A) K+ current traces of GIRK1/2 channels coexpressed with RO4 or mAChR-M2 in HEK293 cells before, during, and after light stimulation (Left) or 10 μM Carb application (Right). Currents were elicited by 500-ms voltage ramps from -100 to +50 mV. (B) Comparison of the GPCR-induced current increase in the presence and absence of 5 nmol PTX. (C) Time course traces of GPCR-mediated activation of GIRK currents. GIRK currents were recorded at -60 mV. (D) Comparison of the time constants of the GPCR-induced GIRK current changes before and after GPCR activation. (E) Ba2+ current traces of P/Q-type Ca2+ channels (α12.1, β1b, and α2δ subunits) coexpressed with RO4 or mAChR-M2 in HEK293 cells before, during, and after light stimulation (Left) or 10 μM Carb application (Right). (F) GPCR-induced depolarizing shift in the voltage dependence of activation curve of P/Q-type Ca2+ currents. Currents were elicited from a holding potential of -60 mV by 5-ms-long, 5-mV voltage steps from -10 to +65 mV. Relative tail currents were plotted against the voltage pulses. (G) Time course traces of GPCR-mediated inhibition of P/Q-type Ca2+ currents. Ba2+ currents were elicited by voltage pulses from -60 to +20 mV and measured every s. (H) Comparison of the time constants of the GPCR-induced P/Q-type channel current changes before and after GPCR activation. Throughout all experiments number in parentheses indicate the number of experiments and statistical significance as indicated (*, P < 0.05; **, P < 0.01, ANOVA).