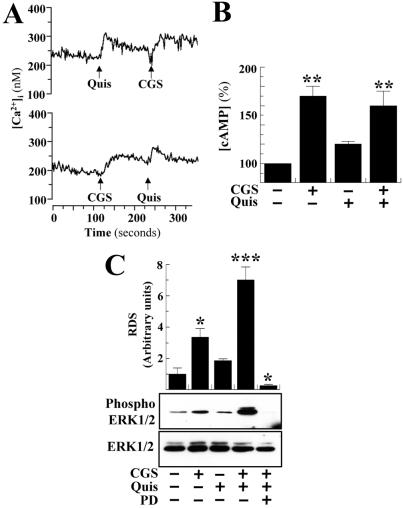
Figure 2.
(A) Ca2+ mobilization, (B) cAMP accumulation, and (C) ERK phosphorylation in A2AR/mGluR5 transiently cotransfected HEK-293 cells. (A) Cells transiently expressing Flag-A2AR plus HA-mGluR5 were preincubated with 0.2 unit/ml adenosine deaminase for 30 min and loaded with FURA-2/AM, and the intracellular Ca2+ mobilization was determined after stimulation with quisqualic acid (100 μM, Quis) or CGS21680 (200 nM, CGS). (B) Cells were preincubated with 0.2 units/ml adenosine deaminase for 30 min and stimulated during 15 min with 100 μM quisqualic acid (Quis) and/or 200 nM CGS21680 (CGS) and the cAMP levels were determined. All values are normalized by using the nontreated transfected cells. The results are expressed as means + SEM (in percent of the values obtained in nontreated transfected cells) of three independent experiments performed in duplicate. Raw data from the different groups analyzed with repeated measures ANOVA followed by Newman–Keuls post hoc comparisons. **, P < 0.01 vs. nontreated transfected cells. (C) Serum-starved cells were preincubated with adenosine deaminase and stimulated with CGS 2160 (200 nM) and/or quisqualic acid (100 μM) for 5 min in the presence or absence of PD98059 (75 nM). The phosphorylation of ERK1/2 was determined by immunoblotting (see Methods). (Lower) Representative ERK assay; (Upper) the intensities of the immunoreactive bands on x-ray film corresponding to ERK1/2 and phospho ERK1/2 protein were measured by densitometric scanning. All values of phosphorylated ERK1/2 were normalized by using ERK1/2 and expressed as means + SEM (in relative densitometric scanning obtained in nontreated transfected cells) of three independent experiments. ANOVA followed by Newman–Keuls post hoc comparisons. * and ***, P < 0.05 and P < 0.001, respectively, versus nontreated transfected cells.