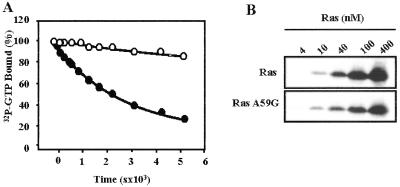
Fig 3.
(A) Comparison of the GTP-hydrolysis rate of wild-type Ras (closed circles) and RasA59G (open circles). The GTP-hydrolysis rate was measured by using the filter-binding assay. Histidine-tagged Ras proteins (40 pM) were loaded with [γ-32P]GTP (25 Ci/mmol; ICN; 1 Ci = 37 GBq) in 40 mM Hepes/100 mM NaCl/10 mM MgCl2/1 mM DTT, pH 8.0/2 mM EDTA at room temperature. After incubation, MgCl2 was added to a final concentration of 20 mM and samples were placed on ice. GTPase reaction was initiated by incubating at 30°C. Aliquots (20 μl) were taken at appropriate times, and the remaining radioactivity was measured on nitrocellulose filter discs (Millipore HAWPO2500) by using a scintillation counter. Results are shown as percentages of radioactivity bound at time 0. Hydrolysis rates (kcat) were calculated by fitting the experimental points to a single exponential by using SIGMA PLOT (SPSS, Chicago) giving values of 2 × 10−4 s−1 and 3.5 × 10−5 s−1 for wild-type Ras and RasA59G, respectively. (B) Binding of RasA59G to the Ras-binding domain (RBD) of the Raf kinase. Glutathione S-transferase-fusion RafRBD was incubated with increasing amounts of wild type (Upper) or A59G (Lower) histidine-tagged Ras loaded with guanosine 5′-O-(3-thiotriphosphate), as indicated. RafRBD/Ras complexes were precipitated with glutathione Sepharose, and the bound material was analyzed by Western blotting with antihistidine antibodies.