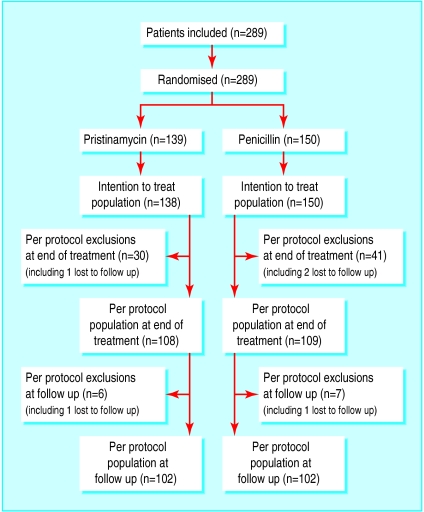
Figure.
Trial regimen (*one patient randomised twice in error but was included only once in efficacy analysis). Exclusions from per protocol at end of treatment were because of non-compliance with treatment, prohibited treatment used during study, discontinuation of treatment due to adverse event, or missing data (patient may have had one or more major protocol violation). Exclusions at follow up were because of missing data or prohibited treatment used during study (patient may have had one or more major protocol violation)