Humans have been using nonsteroid antiinflammatory drugs (NSAIDs) in various forms for more than 3,500 years (1). They are still our favorite medicines. Estimates vary, but it appears, for instance, that each year we consume around 40,000 metric tons of aspirin, equating to about 120 billion aspirin tablets (300 mg is a standard size). In addition, dozens of other NSAIDs and NSAID formulations are available and enthusiastically consumed in most countries. However, despite this long history and large volume of use, we still have an incomplete understanding of how the NSAIDs achieve their actions. Most recently, molecular biology, together with pharmacology, has brought the greatest steps forward in knowledge. It is in this vein that Dan Simmon's group report the discovery of a novel cyclooxygenase (COX) enzyme variant that could be the target of acetaminophen and other analgesic/antipyretic drugs (2).
After 3,500 years, the first real progress in our understanding of the mechanism of the NSAIDs came 30 years ago.
After 3,500 years, the first real progress in our understanding of the mechanism of action of the NSAIDs came 30 years ago, when it was revealed that these chemically varied drugs all reduced the formation of prostaglandins. This ability was associated with inhibition of COX, which converts arachidonic acid to the prostaglandin precursor prostaglandin (PG) H2 (3). The prostanoid family was revealed through associated studies, and PGH2 was shown to be the precursor for prostanoids including PGD2, PGE2, PGF2α, PGI2, and thromboxane A2 (4). Understanding that the NSAIDs inhibited prostanoid formation led to an appreciation of the mechanisms underlying the effects of these drugs. At sites of inflammation, the local production of prostanoids such as PGE2 can sensitize pain nerve endings and increase blood flow, promoting feelings of pain and driving tissue swelling and redness (1, 5). Inhibition of PGE2 formation via the inhibition of local COX could therefore explain the antiinflammatory effects of NSAIDs. Similarly, prostanoids such as PGI2 and PGE2 were found to be protective to the stomach and so inhibition of their formation provided an explanation for the gastrointestinal toxicity associated with prolonged and high-dose use of NSAIDs (1, 5, 6). The inhibition of COX in platelets provided an explanation for the ability of aspirin to reduce blood clotting (7). But still there were a number of questions that remained unanswered through the 1970s and 1980s. For instance, why, when used at similar antiinflammatory doses, were the NSAIDs differently toxic to the gastrointestinal tract (5, 8)? Also, how did acetaminophen fit into this scheme? Did it act by inhibiting COX? Intriguingly, test tube experiments showed that acetaminophen might selectively target the COX present in the brain (9). Could this explain why it was analgesic and antipyretic but not antiinflammatory?
The next great step forward in our understanding of the NSAIDs came in the early 1990s with the demonstration that there were two isoforms of COX: COX-1, which was constitutively expressed, and COX-2, which was inducible (1, 5, 10–13). COX-2 was rapidly up-regulated at inflammatory sites and appeared responsible for the formation of proinflammatory prostanoids. COX-1, meanwhile, appeared to shoulder the responsibility for the production of physiologically relevant prostanoids such as those in the stomach and platelets (1, 5). Pharmacology defined the selectivity of existing NSAIDs on these COX enzymes (1, 5, 14, 15) and played a key role in producing a new generation of COX-2-selective drugs (now selling in vast quantity). These drugs would, it was hoped, be antiinflammatories as good as the traditional NSAIDs but have much reduced toxic side effects, particularly on the gastrointestinal tract (1, 5).
Although providing a much-needed leap in our understanding, the COX-1/-2 model did not appear to explain everything. Even though in inflammatory models COX-2 inhibitors were as active as traditional NSAIDs, worked similarly in both human and animal models of pain, and reduced fever in a similar way to the NSAIDs (1, 5, 16), there were still some confusing issues. For example, the widespread use of the newer generation of COX-2-selective compounds demonstrated that COX-2 also had physiological roles, being involved, for instance, in the maintenance of fluid balance by the kidney (17). The COX-1/-2 model was also not accommodating to the characteristics of acetaminophen: although its antipyretic and analgesic effects might be explained by inhibition of COX-2, why was acetaminophen not antiinflammatory (18)? Dan Simmon's group suggest this is because of the presence of a variant of COX-1, which they have named COX-3, that is especially sensitive to acetaminophen and related compounds (2). If this enzyme were particularly expressed in the brain, could it explain both the characteristics of acetaminophen and Flower and Vane's findings from 30 years ago (9)? It is difficult to produce an unequivocal reply, but let us try to approach an answer by drawing on what we know about acetaminophen and the roles of COX-1 and -2. To begin with, let us consider fever, because pyresis is a more simply modeled process than analgesia. Interestingly, and at odds with Chandrasekharan et al. (2), studies of the prostanoid-producing enzyme underlying pyresis associate it to neither COX-1 protein nor the COX-1 gene. For instance, in mice, it is deletion of the COX-2 but not of the COX-1 gene (which also encodes COX-3) that blunts pyresis (19). In addition, COX-2-selective inhibitors, which will react weakly with the COX-3 enzymatic site, because it is identical to that in COX-1, are as good at reducing fever similarly as traditional NSAIDs (20–23). The fever response has also been clearly associated with a rapid induction of COX-2 expression and an associated increase in PGE2 production (24), with no role for COX-1 or a COX-1 gene product (e.g., COX-3). Finally, the sites of COX-3 expression do not appear to accord well with those sites associated with fever, and we might expect to see the protein present within the hypothalamus (25) rather than the cerebral cortex. All these considerations appear to argue against the COX-3 of Chandrasekharan et al. (2) being the site of the antipyretic actions of NSAIDs and COX-2-selective agents. However, the results from Chandrasekharan et al. could be read as showing that acetaminophen acts at a different site to the other NSAIDs and that more than one COX isoform contributes to the fever response.
Pain is a more difficult process to unravel. As outlined above, prostanoids produced at sites of inflammation can sensitize nerve endings and so promote the localized feelings of pain associated with inflammatory events and tissue injury (26). These prostanoids can be produced by COX-2 induced by the local inflammatory processes. How then does acetaminophen bring about its analgesic effects, because it is not a peripheral antiinflammatory? With the discovery of COX-2, new efforts have been made to comprehend the roles of prostanoids within the central nervous system (CNS). It appears that COX-2 is constitutively expressed in the CNS and also rapidly up-regulated to reinforce pain perception (27, 28). Could acetaminophen act on this CNS enzyme? COX-2-selective inhibitors appear to produce analgesic responses at least as good as traditional NSAIDs in inflammatory, dental, or postoperative pain (29–31). These activities would support, as above, a role for COX-2 or a COX-2 variant rather than a COX-1 variant. But maybe acetaminophen targets an additional enzyme. We know there may be both redundancy and compensatory increases in COX enzymes; for instance, COX-2 may be up-regulated when COX-1 is inhibited in the stomach (32). Furthermore, Dan Simmon's group has previously proposed the existence of an isoform of COX-2 that is particularly sensitive to acetaminophen (33, 34).
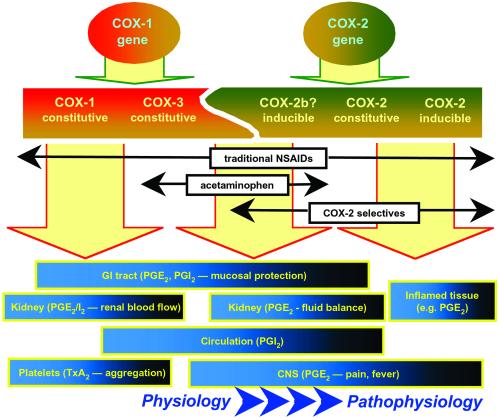
From the above, it seems that the most significant implication of the study of Chandrasekharan et al. (2) is that multiple COX isoenzymes could be derived from just two distinct genes providing a COX continuum of enzymes and products (Fig. 1). Could the presence of multiple isoforms of COX-1 and -2 explain why there are so many different NSAIDs on the market and why different patients appear to benefit from different types of NSAID? If we express variants of COX-1 and -2, could different drugs inhibit different variants to different extents? Indeed, could these findings help us understand some of the side effects of NSAIDs and COX-2-selective inhibitors? For example, much recent attention has been paid to the ability of COX-2-selective inhibitors to reduce circulating PGI2 levels and to the hypothesis that this could be linked to an increase in thrombotic risk (35). Interestingly, acetaminophen also reduces circulating PGI2 levels (36), yet it is not a selective COX-2 inhibitor. Could this PGI2 actually be formed by the COX-3 that Chandrasekharan et al. (2) have found in the heart and aorta? Or could varied products from just two distinct genes provide a family of COX proteins with overlapping contributions to prostanoid production throughout the body? Might both inducible COX-2 and COX-3 be involved in the fever response, whereas both constitutive COX-2 and COX-3 contribute to the circulatory production of PGI2? We know that both COX-1 and -2 have constitutive roles in the kidney. All this has yet to be understood, but as we continue to push toward an understanding, it is important to note that Chandrasekharan et al.'s study also tells us that modifications in regions of the COX molecule other than the enzymatic site may influence inhibitor selectivity, possibly by affecting the interplay with other active intracellular species (37). In terms of our 3,500-year quest to understand the mechanism of action of the NSAIDs, Dan Simmon's group has provided another significant step forward.
Figure 1.
A COX continuum? Two distinct genes for COX-1 and -2 may give rise to a number of constitutive and inducible COX proteins with overlapping functions. Considering prostanoid production by a COX continuum may help us appreciate which enzymes underlie prostanoid production in different tissues as well as the actions of traditional NSAIDs, newer COX-2-selective drugs, and acetaminophen (“COX-2b,” as suggested in ref. 33; COX-3, as suggested in ref. 2).
Footnotes
See companion article on page 13926.
References
- 1.Vane J R. J Physiol Pharmacol. 2000;51:573–586. [PubMed] [Google Scholar]
- 2.Chandrasekharan N V, Dai H, Roos L T, Evanson N K, Tomsik J, Elton T S, Simmons D L. Proc Natl Acad Sci USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vane J R. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson B. Biosci Rep. 1983;3:791–813. doi: 10.1007/BF01133779. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell J A, Warner T D. Br J Pharmacol. 1999;128:1121–1132. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe M M, Lichtenstein D R, Singh G. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 7.Patrono C. Am J Med. 2001;110:62S–65S. doi: 10.1016/s0002-9343(00)00645-8. [DOI] [PubMed] [Google Scholar]
- 8.Henry D, Lim L L, Garcia Rodriguez L A, Perez Gutthann S, Carson J L, Griffin M, Savage R, Logan R, Moride Y, Hakwey C, et al. Br Med J. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flower R J, Vane J R. Nature. 1972;240:410–411. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- 10.Kujubu D A, Fletcher B S, Varnum B C, Lim R W, Herschman H R. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 11.Xie W L, Chipman J G, Robertson D L, Erikson R L, Simmons D L. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fletcher B S, Kujubu D A, Perrin D M, Herschman H R. J Biol Chem. 1992;267:4338–4344. [PubMed] [Google Scholar]
- 13.O'Banion M K, Winn V D, Young D A. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell J A, Akarasereenont P, Thiemermann C, Flower R J, Vane J R. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner T D, Giuliano F, Vojnovic I, Bukasa A, Mitchell J A, Vane J R. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turini M E, DuBois R N. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 17.Khan K N, Paulson S K, Verburg K M, Lefkowith J B, Maziasz T J. Kidney Int. 2002;61:1210–1219. doi: 10.1046/j.1523-1755.2002.00263.x. [DOI] [PubMed] [Google Scholar]
- 18.Prescott L F. Am J Ther. 2000;7:143–147. [PubMed] [Google Scholar]
- 19.Li S, Wang Y, Matsumura K, Ballou L R, Morham S G, Blatteis C M. Brain Res. 1999;825:86–94. doi: 10.1016/s0006-8993(99)01225-1. [DOI] [PubMed] [Google Scholar]
- 20.Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner M F. Nat Struct Biol. 1996;3:927–933. doi: 10.1038/nsb1196-927. [DOI] [PubMed] [Google Scholar]
- 21.Kurumbail R G, Stevens A M, Gierse J K, McDonald J J, Stegeman R A, Pak J Y, Gildehaus D, Miyashiro J M, Penning T D, Seibert K, et al. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 22.Riendeau D, Percival M D, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Falgueyret J P, Ford-Hutchinson A W, et al. Br J Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riendeau D, Percival M D, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret J P, Friesen R W, Gordon R, Greig G, et al. J Pharmacol Exp Ther. 2001;296:558–566. [PubMed] [Google Scholar]
- 24.Cao C, Matsumura K, Yamagata K, Watanabe Y. Brain Res. 1996;733:263–272. doi: 10.1016/0006-8993(96)00575-6. [DOI] [PubMed] [Google Scholar]
- 25.Aronoff D M, Neilson E G. Am J Med. 2001;111:304–315. doi: 10.1016/s0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S M, Brahim J S, Rowan J, Kent A, Dionne R A. Clin Pharmacol Ther. 2002;72:175–183. doi: 10.1067/mcp.2002.126501. [DOI] [PubMed] [Google Scholar]
- 27.Samad T A, Moore K A, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre J V, Woolf C J. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 28.Svensson C I, Yaksh T L. Annu Rev Pharmacol Toxicol. 2002;42:553–583. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- 29.Dougados M, Behier J M, Jolchine I, Calin A, van der Heijde D, Oliveri I, Zeidler H, Herman H. Arthritis Rheum. 2001;44:180–185. doi: 10.1002/1529-0131(200101)44:1<180::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Chang D J, Desjardins P J, Chen E, Polis A B, McAvoy M, Mockoviak S H, Geba G P. Clin Ther. 2002;24:490–503. doi: 10.1016/s0149-2918(02)85126-8. [DOI] [PubMed] [Google Scholar]
- 31.Camu F, Beecher T, Recker D P, Verburg K M. Am J Ther. 2002;9:43–51. doi: 10.1097/00045391-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka A, Araki H, Hase S, Komoike Y, Takeuchi K. Aliment Pharmacol Ther. 2002;16:90–101. doi: 10.1046/j.1365-2036.16.s2.22.x. [DOI] [PubMed] [Google Scholar]
- 33.Simmons D L, Botting R M, Robertson P M, Madsen M L, Vane J R. Proc Natl Acad Sci USA. 1999;96:3275–3280. doi: 10.1073/pnas.96.6.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Botting R. J Physiol Pharmacol. 2000;51:609–618. [PubMed] [Google Scholar]
- 35.FitzGerald G A. Am J Cardiol. 2002;89:26D–32D. doi: 10.1016/s0002-9149(02)02234-8. [DOI] [PubMed] [Google Scholar]
- 36.Green K, Drvota V, Vesterqvist O. Prostaglandins. 1989;37:311–315. doi: 10.1016/0090-6980(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 37.Boutaud O, Aronoff D M, Richardson J H, Marnett L J, Oates J A. Proc Natl Acad Sci USA. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]