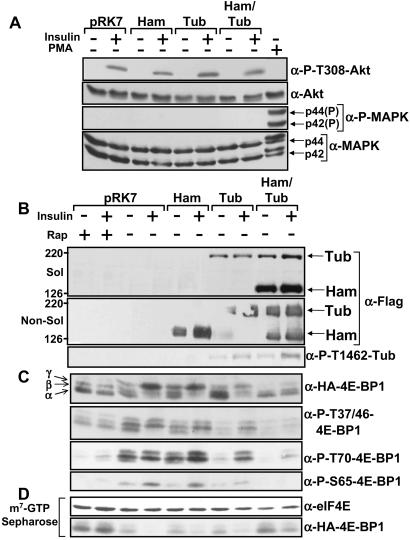
Figure 1.
Hamartin and tuberin inhibits PI3K-dependent 4E-BP1 phosphorylation. HEK293E cells coexpressing Flag-tagged hamartin (Ham) and tuberin (Tub), where indicated, with HA-tagged 4E-BP1 were serum-starved and pretreated with 20 nM rapamycin (rap) for 30 min before being stimulated with insulin (100 nM) or PMA (100 ng/ml) for 30 min, where indicated. (A) The levels and Thr-308 phosphorylation of Akt and the levels and phosphorylation of the MAPK isoforms (p44 and p42) were determined. (B) Hamartin and tuberin protein levels were accessed from the cell lysates (Sol) and the insoluble fraction (Non-Sol) as described in Materials and Methods by using the anti-Flag antibody. Thr-1462 tuberin phosphorylation was analyzed by using a tuberin Thr-1462 phospho-specific antibody. (C) The phosphorylation of exogenous 4E-BP1 was determined with an anti-HA antibody and phospho-specific antibodies for 4E-BP1 at Thr-37 and/or 46, Ser-65, and Thr-70, as indicated. The α-, β-, and γ-species of 4E-BP1 are labeled accordingly. (D) Cell extracts were subjected to affinity chromatography on m7GTP-Sepharose, as described in Materials and Methods. The levels of eIF4E and exogenous 4E-BP1 that was copurified were determined.