Figure 2.
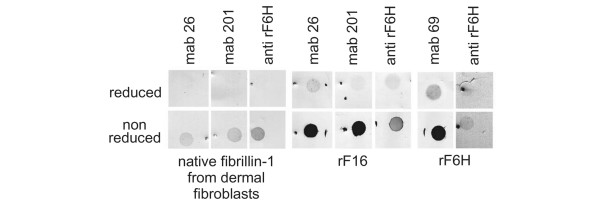
Immunoreactive analysis of fibrillin-1 antibodies against recombinant fibrillin-1 polypeptides and against native fibrillin-1. Purified recombinant amino-terminal (rF16) and carboxy-terminal (rF6H) halves of human fibrillin-1 (2 μg of each) or 1 ml of conditioned medium (containing less than about 0.2 μg of fibrillin-1) produced by human dermal fibroblasts were transferred to nitrocellulose membranes with (upper panel) or without (lower panel) previous reduction by dithiothreitol. Nitrocellulose membranes were probed with a polyclonal antibody against rF6H (anti-rF6H) or with monoclonal antibodies (mAbs) 26, 201, and 69 directed against rF16 and rF6H. The dot-blots show that the binding of all antibodies depends markedly on the presence of disulfide bonds, which are crucial for the proper folding of epitopes in both native fibrillin-1 and the recombinant fragments.
