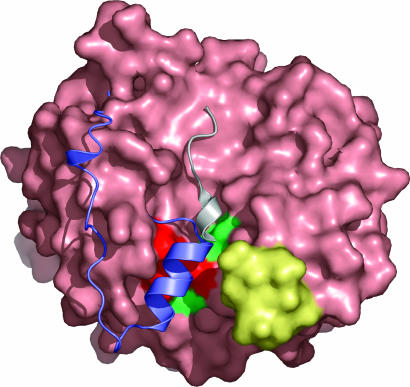
Fig. 4.
Molecular interaction surface of RsbR. The molecular surface of the N-RsbR dimer is light pink. The Z-helix and preceding ≈20 aa (both in purple) of HemAT (31) and the KaiC-derived peptide (gray) of the KaiA–KaiC complex (40) are displayed after the superimposition of HemAT and KaiA on the dimer of N-RsbR. Both of these parts of structure are found to overlap in a groove formed on the surface of N-RsbR, which is flanked on one side by the C–D corner (yellow), mutations in which, in Vitreoscilla hemoglobin, affect the binding of the flavin-binding domain of 2,4-dinitrotoluene dioxygenase (51). The positions of E60, K82, and E126, mutation of which affects RsbT binding to RsbR–RsbS, and T86 and N129, mutation of which does not affect RsbT binding to RsbR–RsbS, are highlighted on the surface of N-RsbR in red and green, respectively.