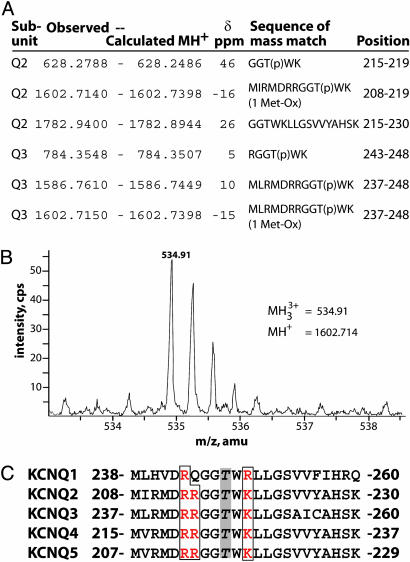
Fig. 3.
Analysis of a potential phosphorylation site in the S4-S5 loop. (A) A set of overlapping peptide mass matches implicates a conserved threonine in the S4-S5 loop as a site of phosphorylation. The observed and calculated monoisotopic (MH+) masses of the KCNQ subunit peptides, the error δ (in parts per million), the peptide sequence, and position in each subunit are indicated. (B) MS spectrum showing low intensity triply charged ion with m/z (mass normalized by charge) of 534.91 atomic mass units (amu), detected in tryptic digest of KCNQ2. This corresponds to monoisotopic mass of 1602.714 amu, a close match for the residues 208-219 of KCNQ2 if Thr-217 is phosphorylated. (C) The KCNQ S4-S5 loop sequence is highly conserved. All family members contain a threonine corresponding to the phosphorylated threonines, Thr-217 of KCNQ2 and Thr-246 of KCNQ3, and surrounding basic residues.