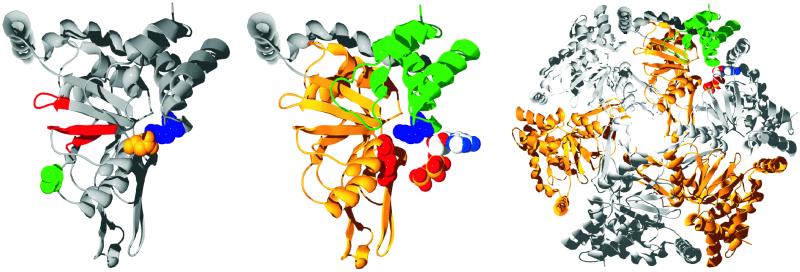
Figure 1.
(Left) The structure of R. capsulatus BchI is shown as a ribbon diagram. The Walker AB motif and the sensor 1 region are colored red, and the residues that are changed in the mutants are shown as space-filled residues, with L111 colored green, D207 colored gold, and R289 colored blue. (Center) R. capsulatus BchI folds into two domains connected by a long helical region colored in gray. The N-terminal is gold, and the C-terminal domain is green. Two important residues in AAA+ proteins, the arginine finger and the sensor arginine, are shown as space-filled residues and are colored red and blue, respectively. An ATP from a neighboring subunit is modeled next to BchI. (Right) From the BchI hexamer, it is clearly seen that the mutated residues lie at the interface between two neighboring subunits. All images were made with swiss-pdbviewer (33), rendered with pov-ray (Persistence of Vision, Indianapolis), and colored by corel draw (Corel, Ottawa).