Abstract
PURPOSE: First, to study the cellular mechanisms of acquired color vision loss in retinal detachment and diabetic retinopathy. Second, to learn why, in glaucoma, the type of color vision deficit that is observed is more characteristic of a retinal injury than it is of an optic neuropathy. Third, to test a hypothesis of photoreceptor-induced, ganglion cell death in glaucoma. METHODS: Various histologic techniques were employed to distinguish the L/M-cones (long/medium wavelength-sensitive cones, or red/green sensitive cones) from the S-cones (short wavelength-sensitive cones, or blue sensitive cones) in humans and monkeys with retinal detachment, humans with diabetic retinopathy, and both humans and monkeys with glaucoma. To test if the photoreceptors were contributing to ganglion cell death, laser photocoagulation was used in a experimental model of glaucoma to focally eliminate the photoreceptors. As a control, optic nerve transection was done following retinal laser photocoagulation in one animal. RESULTS: Selective and widespread loss of the S-cones was found in retinal detachment as well as diabetic retinopathy. By contrast, in human as well as experimental glaucoma, marked swelling of the L/M-cones was the predominant histopathologic feature. Retinal laser photocoagulation followed by experimental glaucoma resulted in selective protection of ganglion cells overlying the laser spots. This was not seen with retinal laser photocoagulation by optic nerve transection. CONCLUSIONS: In retinal detachment and diabetic retinopathy, acquired tritan-like color vision loss could be caused, or contributed to, by selective loss of the S-cones. Both L- and M-cones are affected in glaucoma, which is also consistent with a tritan-like deficit. Although not a therapeutic option, protection of ganglion cells by retinal laser in experimental glaucoma is consistent with an hypothesis of anterograde, photoreceptor-induced, ganglion cell death.
Full text
PDF




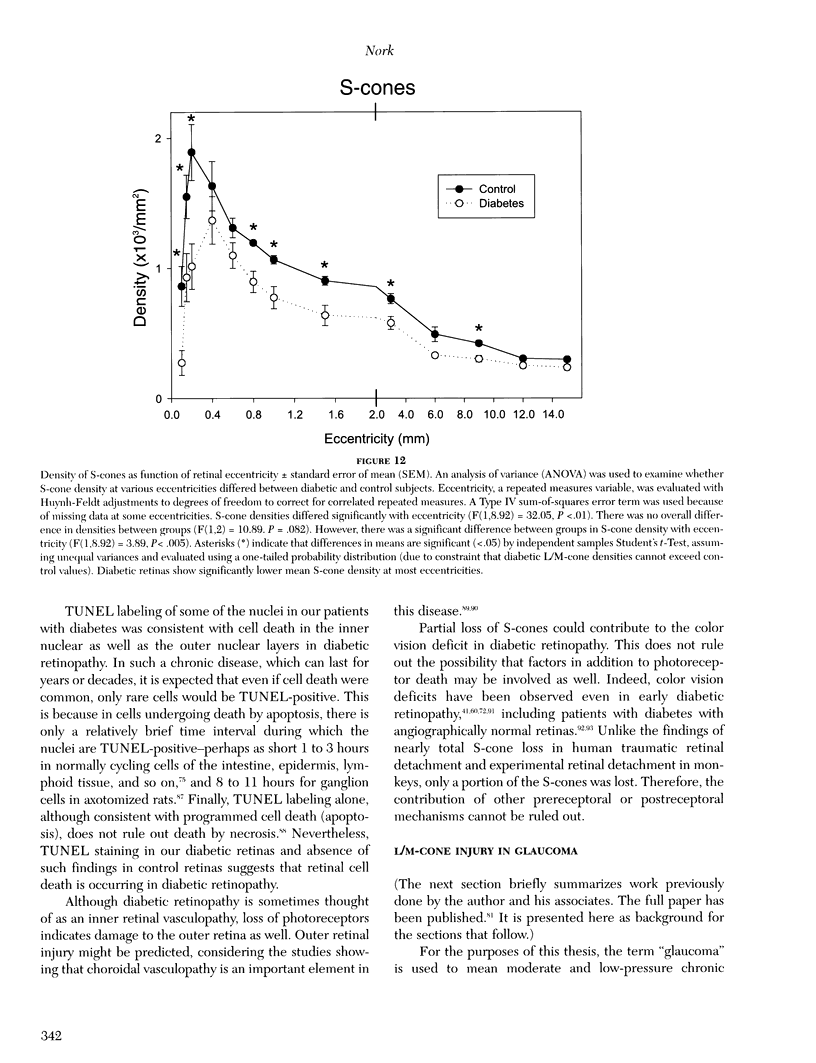
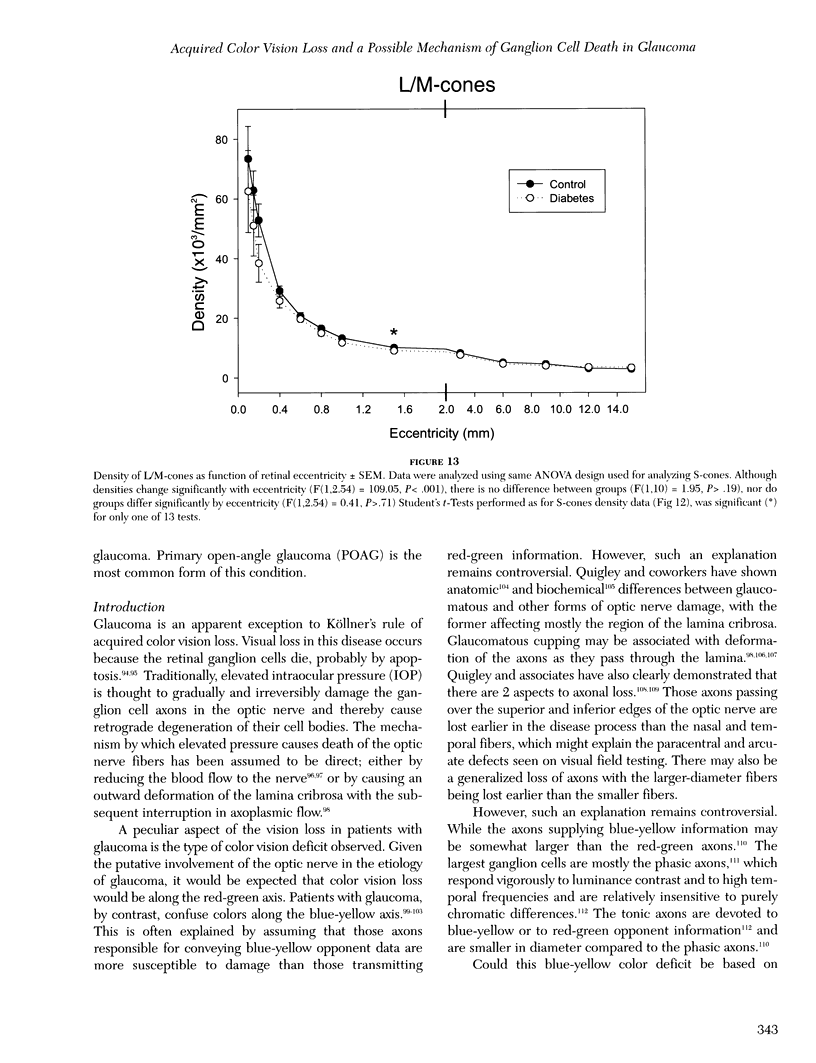
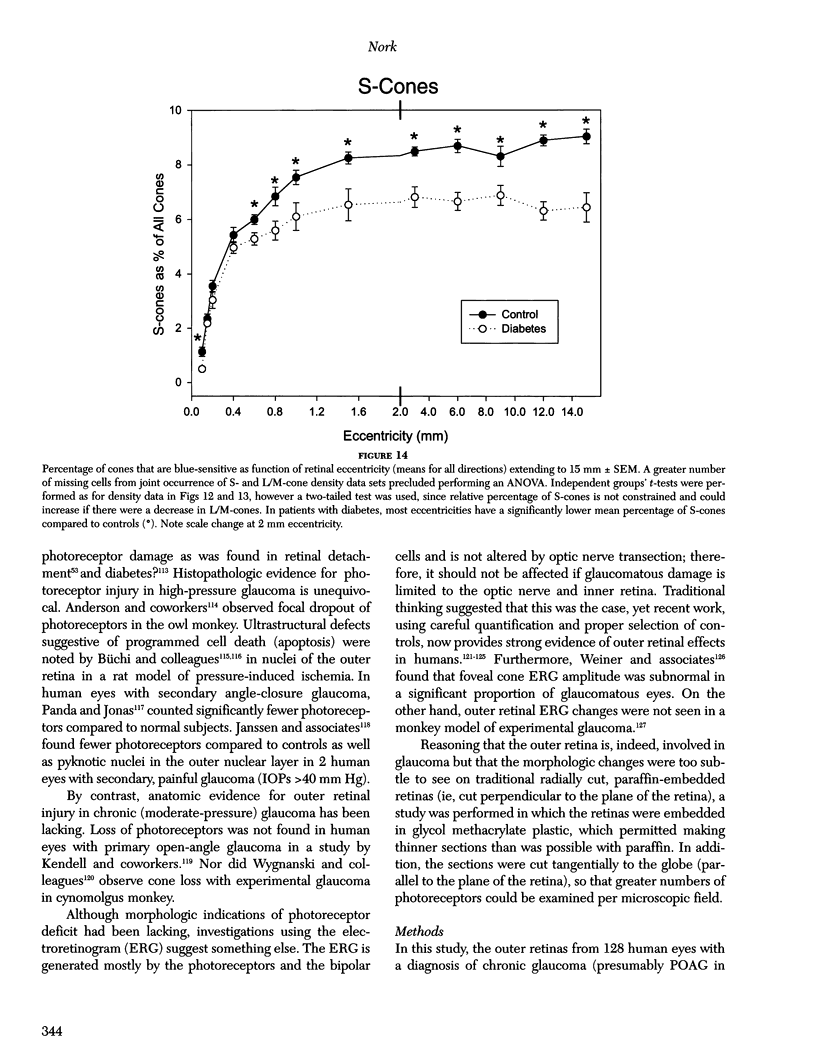
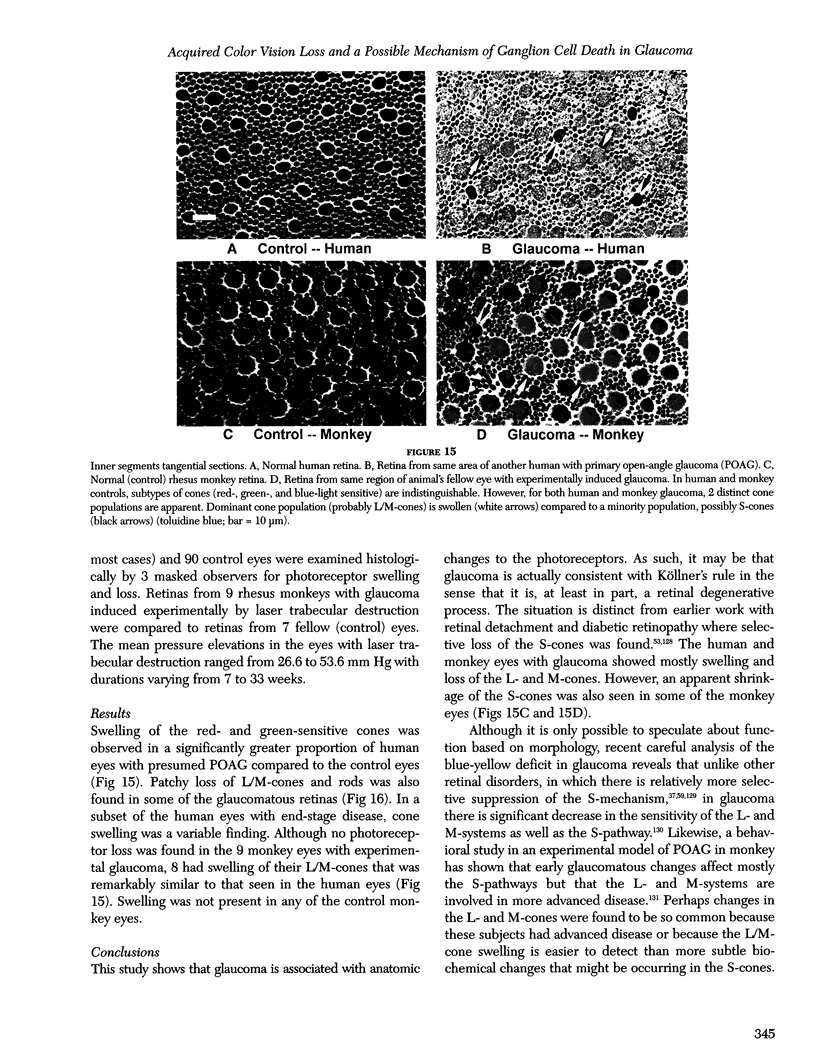











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. J. Chromatic and luminosity processing in retinal disease. Am J Optom Physiol Opt. 1982 Dec;59(12):954–960. doi: 10.1097/00006324-198212000-00004. [DOI] [PubMed] [Google Scholar]
- Ahnelt P. K., Kolb H., Pflug R. Identification of a subtype of cone photoreceptor, likely to be blue sensitive, in the human retina. J Comp Neurol. 1987 Jan 1;255(1):18–34. doi: 10.1002/cne.902550103. [DOI] [PubMed] [Google Scholar]
- Alm A., Bill A. Blood flow and oxygen extraction in the cat uvea at normal and high intraocular pressures. Acta Physiol Scand. 1970 Sep;80(1):19–28. doi: 10.1111/j.1748-1716.1970.tb04765.x. [DOI] [PubMed] [Google Scholar]
- Alm A., Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973 Jan 1;15(1):15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Alvis D. L. Electroretinographic changes in controlled chronic open-angle glaucoma. Am J Ophthalmol. 1966 Jan;61(1):121–131. doi: 10.1016/0002-9394(66)90756-2. [DOI] [PubMed] [Google Scholar]
- Anderson D. H., Guérin C. J., Erickson P. A., Stern W. H., Fisher S. K. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986 Feb;27(2):168–183. [PubMed] [Google Scholar]
- Anderson D. R., Davis E. B. Sensitivities of ocular tissues to acute pressure-induced ischemia. Arch Ophthalmol. 1975 Apr;93(4):267–274. doi: 10.1001/archopht.1975.01010020277006. [DOI] [PubMed] [Google Scholar]
- Baxter G. M., Williamson T. H., McKillop G., Dutton G. N. Color Doppler ultrasound of orbital and optic nerve blood flow: effects of posture and timolol 0.5%. Invest Ophthalmol Vis Sci. 1992 Mar;33(3):604–610. [PubMed] [Google Scholar]
- Becker B. Diabetes mellitus and primary open-angle glaucoma. The XXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1971 Jan;71(1 Pt 1):1–16. doi: 10.1016/0002-9394(71)91088-9. [DOI] [PubMed] [Google Scholar]
- Birch-Cox J. Defective colour vision in diabetic retinopathy before and after laser photocoagulation. Mod Probl Ophthalmol. 1978;19:326–329. [PubMed] [Google Scholar]
- Brandstätter J. H., Hartveit E., Sassoè-Pognetto M., Wässle H. Expression of NMDA and high-affinity kainate receptor subunit mRNAs in the adult rat retina. Eur J Neurosci. 1994 Jul 1;6(7):1100–1112. doi: 10.1111/j.1460-9568.1994.tb00607.x. [DOI] [PubMed] [Google Scholar]
- Bresnick G. H., Condit R. S., Palta M., Korth K., Groo A., Syrjala S. Association of hue discrimination loss and diabetic retinopathy. Arch Ophthalmol. 1985 Sep;103(9):1317–1324. doi: 10.1001/archopht.1985.01050090069034. [DOI] [PubMed] [Google Scholar]
- Burton T. C. Recovery of visual acuity after retinal detachment involving the macula. Trans Am Ophthalmol Soc. 1982;80:475–497. [PMC free article] [PubMed] [Google Scholar]
- Butt Z., O'Brien C., McKillop G., Aspinall P., Allan P. Color Doppler imaging in untreated high- and normal-pressure open-angle glaucoma. Invest Ophthalmol Vis Sci. 1997 Mar;38(3):690–696. [PubMed] [Google Scholar]
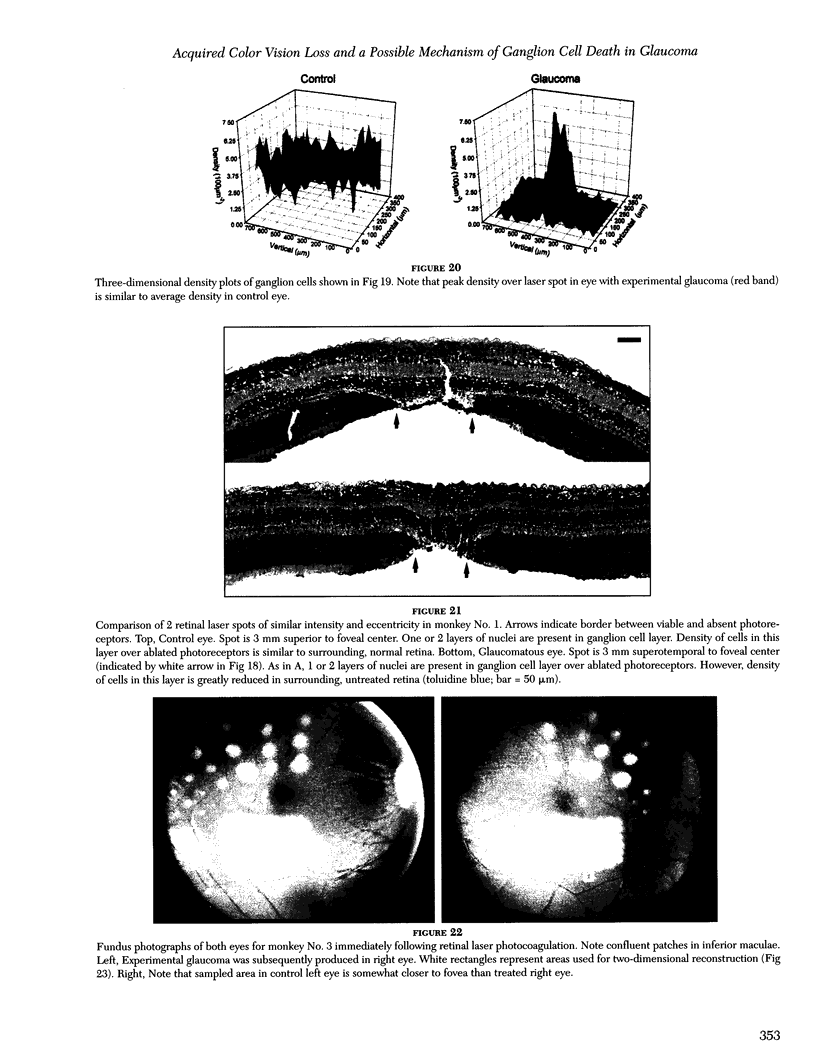
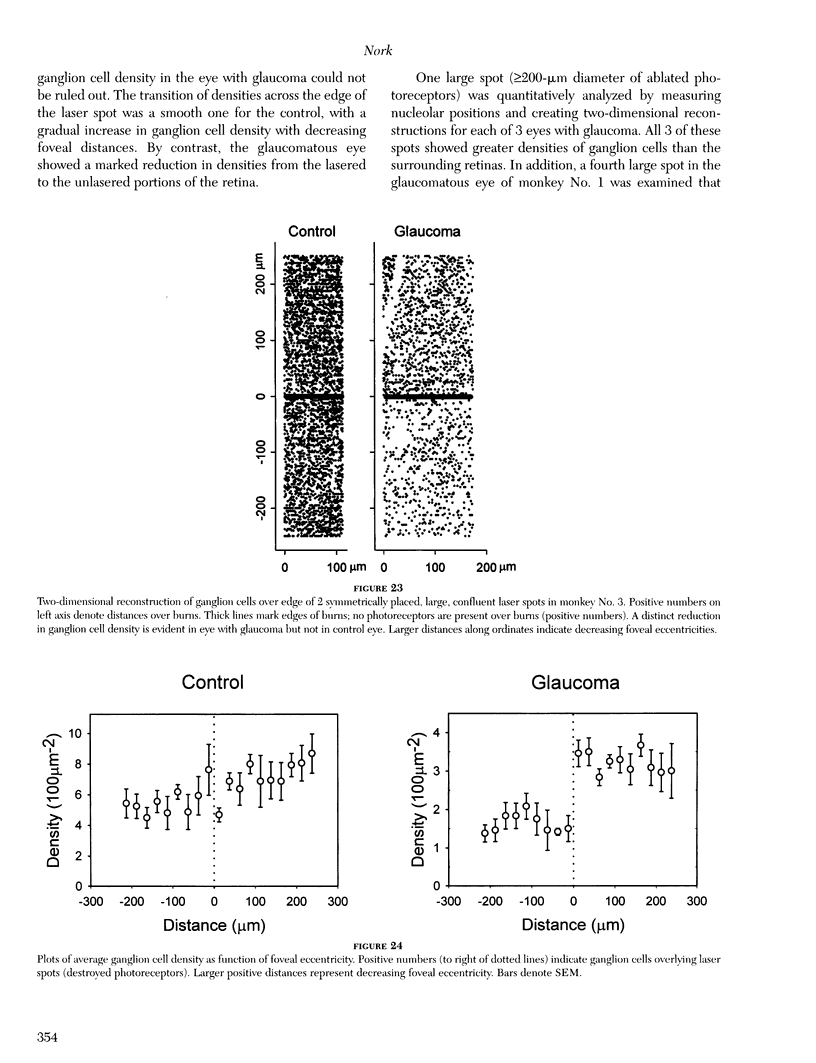
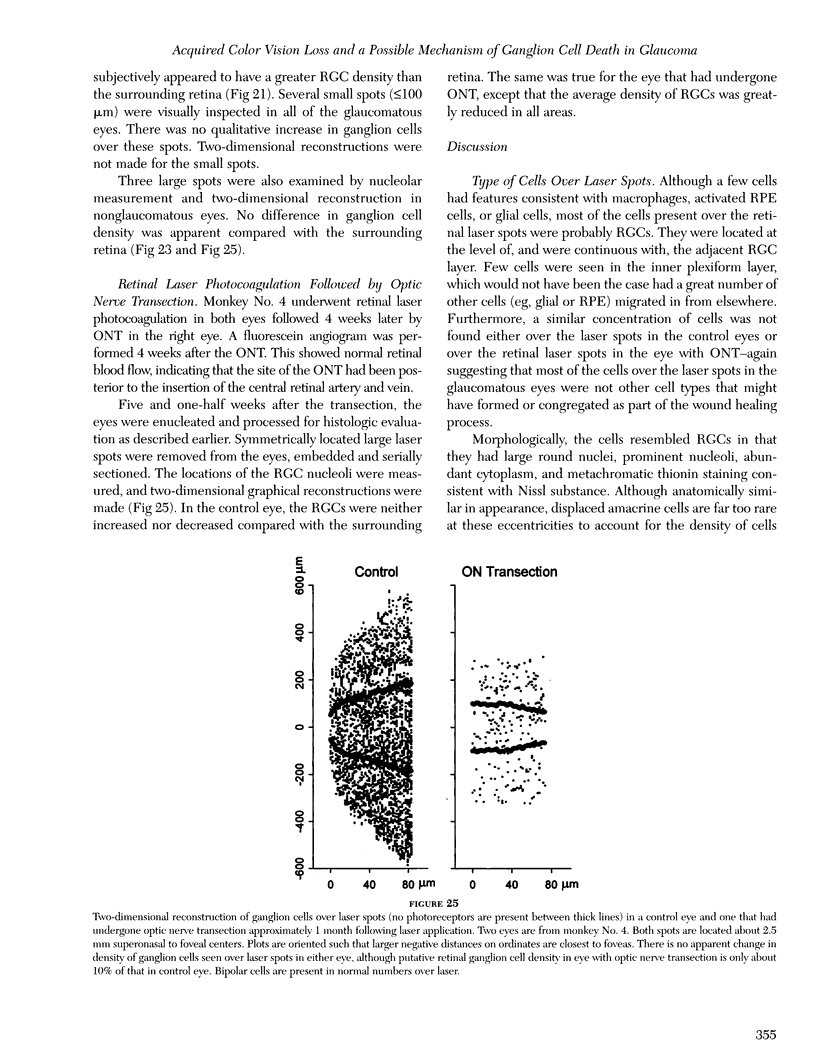
- Büchi E. R. Cell death in rat retina after pressure-induced ischaemia-reperfusion insult: electron microscopic study. II. Outer nuclear layer. Jpn J Ophthalmol. 1992;36(1):62–68. [PubMed] [Google Scholar]
- Büchi E. R. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992 Oct;55(4):605–613. doi: 10.1016/s0014-4835(05)80173-3. [DOI] [PubMed] [Google Scholar]
- Cao J., McLeod S., Merges C. A., Lutty G. A. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998 May;116(5):589–597. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- Cellini M., Possati G. L., Caramazza N., Caramazza R. Colour Doppler analysis of the choroidal circulation in chronic open-angle glaucoma. Ophthalmologica. 1996;210(4):200–202. doi: 10.1159/000310708. [DOI] [PubMed] [Google Scholar]
- Chang G. Q., Hao Y., Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993 Oct;11(4):595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- Chiou G. C., Chen Y. J. Effects of antiglaucoma drugs on ocular blood flow in ocular hypertensive rabbits. J Ocul Pharmacol. 1993 Spring;9(1):13–24. doi: 10.1089/jop.1993.9.13. [DOI] [PubMed] [Google Scholar]
- Chisholm I. A., McClure E., Foulds W. S. Functional recovery of the retina after retinal detachment. Trans Ophthalmol Soc U K. 1975 Apr;95(1):167–172. [PubMed] [Google Scholar]
- Collignon-Brach J. Longterm effect of topical beta-blockers on intraocular pressure and visual field sensitivity in ocular hypertension and chronic open-angle glaucoma. Surv Ophthalmol. 1994 May;38 (Suppl):S149–S155. doi: 10.1016/0039-6257(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Allen K. A. Topography of ganglion cells in human retina. J Comp Neurol. 1990 Oct 1;300(1):5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Sloan K. R., Meyers D. Computer methods for sampling, reconstruction, display and analysis of retinal whole mounts. Vision Res. 1989;29(5):529–540. doi: 10.1016/0042-6989(89)90039-4. [DOI] [PubMed] [Google Scholar]
- DeMonasterio F. M., Schein S. J., McCrane E. P. Staining of blue-sensitive cones of the macaque retina by a fluorescent dye. Science. 1981 Sep 11;213(4513):1278–1281. doi: 10.1126/science.7268439. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Krauskopf J., Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Polo A., Aigner L. J., Dunn R. J., Bray G. M., Aguayo A. J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998 Mar 31;95(7):3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diddie K. R., Ernest J. T. The effect of photocoagulation on the choroidal vasculature and retinal oxygen tension. Am J Ophthalmol. 1977 Jul;84(1):62–66. doi: 10.1016/0002-9394(77)90325-7. [DOI] [PubMed] [Google Scholar]
- Didenko V. V., Hornsby P. J. Presence of double-strand breaks with single-base 3' overhangs in cells undergoing apoptosis but not necrosis. J Cell Biol. 1996 Dec;135(5):1369–1376. doi: 10.1083/jcb.135.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K., Hemilä S., Kalamkarov G., Koskelainen A., Shevchenko T. Rod phototransduction modulated by bicarbonate in the frog retina: roles of carbonic anhydrase and bicarbonate exchange. J Physiol. 1990 Jul;426:297–316. doi: 10.1113/jphysiol.1990.sp018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drance S. M. A comparison of the effects of betaxolol, timolol, and pilocarpine on visual function in patients with open-angle glaucoma. J Glaucoma. 1998 Aug;7(4):247–252. doi: 10.1097/00061198-199808000-00006. [DOI] [PubMed] [Google Scholar]
- Dreyer E. B., Zhang D., Lipton S. A. Transcriptional or translational inhibition blocks low dose NMDA-mediated cell death. Neuroreport. 1995 Apr 19;6(6):942–944. doi: 10.1097/00001756-199504190-00029. [DOI] [PubMed] [Google Scholar]
- Dreyer E. B., Zurakowski D., Schumer R. A., Podos S. M., Lipton S. A. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996 Mar;114(3):299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., LaVail M. M., LaVail J. H. Assessment of possible transneuronal changes in the retina of rats with inherited retinal dystrophy: cell size, number, synapses, and axonal transport by retinal ganglion cells. J Comp Neurol. 1984 Feb 10;223(1):22–34. doi: 10.1002/cne.902230103. [DOI] [PubMed] [Google Scholar]
- FRANCOIS J., VERRIEST G. Les dyschromatopsies acquises dans le glancome primaire. Ann Ocul (Paris) 1959 Mar;192(3):191–199. [PubMed] [Google Scholar]
- Fazio D. T., Heckenlively J. R., Martin D. A., Christensen R. E. The electroretinogram in advanced open-angle glaucoma. Doc Ophthalmol. 1986 Jun 16;63(1):45–54. doi: 10.1007/BF00153011. [DOI] [PubMed] [Google Scholar]
- Fishman G. A., Krill A. E., Fishman M. Acquired color defects in patients with open-angle glaucoma and ocular hypertension. Mod Probl Ophthalmol. 1974;13(0):335–338. [PubMed] [Google Scholar]
- Frishman L. J., Shen F. F., Du L., Robson J. G., Harwerth R. S., Smith E. L., 3rd, Carter-Dawson L., Crawford M. L. The scotopic electroretinogram of macaque after retinal ganglion cell loss from experimental glaucoma. Invest Ophthalmol Vis Sci. 1996 Jan;37(1):125–141. [PubMed] [Google Scholar]
- Fryczkowski A. W., Sherman M. D., Walker J. Observations on the lobular organization of the human choriocapillaris. Int Ophthalmol. 1991 Mar;15(2):109–120. doi: 10.1007/BF00224463. [DOI] [PubMed] [Google Scholar]
- Gaasterland D., Kupfer C. Experimental glaucoma in the rhesus monkey. Invest Ophthalmol. 1974 Jun;13(6):455–457. [PubMed] [Google Scholar]
- Garcia-Valenzuela E., Gorczyca W., Darzynkiewicz Z., Sharma S. C. Apoptosis in adult retinal ganglion cells after axotomy. J Neurobiol. 1994 Apr;25(4):431–438. doi: 10.1002/neu.480250408. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnering R. S., Dortzbach R. K., Erickson K. A., Kaufman P. L. The cynomolgus monkey as a model for orbital research. II. Anatomic effects of lateral orbitotomy. Curr Eye Res. 1984 Apr;3(4):541–555. doi: 10.3109/02713688409003054. [DOI] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968 Dec;199(3):533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S. L., Klistorner A. Electrophysiology: a review of signal origins and applications to investigating glaucoma. Aust N Z J Ophthalmol. 1998 Feb;26(1):71–85. doi: 10.1046/j.1440-1606.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- Greenstein V. C., Halevy D., Zaidi Q., Koenig K. L., Ritch R. H. Chromatic and luminance systems deficits in glaucoma. Vision Res. 1996 Feb;36(4):621–629. doi: 10.1016/0042-6989(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Greenstein V. C., Hood D. C., Campbell C. J. The use of a flash-on-flash paradigm to assess sensitivity changes due to retinal disease. Invest Ophthalmol Vis Sci. 1982 Jul;23(1):102–112. [PubMed] [Google Scholar]
- Greenstein V. C., Hood D. C., Ritch R., Steinberger D., Carr R. E. S (blue) cone pathway vulnerability in retinitis pigmentosa, diabetes and glaucoma. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1732–1737. [PubMed] [Google Scholar]
- Greenstein V. C., Shapiro A., Hood D. C., Zaidi Q. Chromatic and luminance sensitivity in diabetes and glaucoma. J Opt Soc Am A Opt Image Sci Vis. 1993 Aug;10(8):1785–1791. doi: 10.1364/josaa.10.001785. [DOI] [PubMed] [Google Scholar]
- Greenstein V. C., Shapiro A., Zaidi Q., Hood D. C. Psychophysical evidence for post-receptoral sensitivity loss in diabetics. Invest Ophthalmol Vis Sci. 1992 Sep;33(10):2781–2790. [PubMed] [Google Scholar]
- Greenstein V. C., Thomas S. R., Blaustein H., Koenig K., Carr R. E. Effects of early diabetic retinopathy on rod system sensitivity. Optom Vis Sci. 1993 Jan;70(1):18–23. doi: 10.1097/00006324-199301000-00005. [DOI] [PubMed] [Google Scholar]
- Greenstein V., Sarter B., Hood D., Noble K., Carr R. Hue discrimination and S cone pathway sensitivity in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1990 Jun;31(6):1008–1014. [PubMed] [Google Scholar]
- Grützner P., Schleicher S. Acquired color vision defects in glaucoma patients. Mod Probl Ophthalmol. 1972;11:136–140. [PubMed] [Google Scholar]
- Gundry M. F., Davies E. W. Recovery of visual acuity after retinal detachment surgery. Am J Ophthalmol. 1974 Mar;77(3):310–314. doi: 10.1016/0002-9394(74)90735-1. [DOI] [PubMed] [Google Scholar]
- Guérin C. J., Anderson D. H., Fariss R. N., Fisher S. K. Retinal reattachment of the primate macula. Photoreceptor recovery after short-term detachment. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1708–1725. [PubMed] [Google Scholar]
- Gündüz K., Arden G. B., Perry S., Weinstein G. W., Hitchings R. A. Color vision defects in ocular hypertension and glaucoma. Quantification with a computer-driven color television system. Arch Ophthalmol. 1988 Jul;106(7):929–935. doi: 10.1001/archopht.1988.01060140075028. [DOI] [PubMed] [Google Scholar]
- HURVICH L. M., JAMESON D. Some quantitative aspects of an opponent-colors theory. II. Brightness, saturation, and hue in normal and dichromatic vision. J Opt Soc Am. 1955 Aug;45(8):602–616. doi: 10.1364/josa.45.000602. [DOI] [PubMed] [Google Scholar]
- Hansson H. P. Histochemical demonstration of carbonic anhydrase activity in some epithelia noted for active transport. Acta Physiol Scand. 1968 Aug;73(4):427–434. doi: 10.1111/j.1365-201x.1968.tb10882.x. [DOI] [PubMed] [Google Scholar]
- Hansson H. P. Histochemical demonstration of carbonic anhydrase activity. Histochemie. 1967;11(2):112–128. doi: 10.1007/BF00571716. [DOI] [PubMed] [Google Scholar]
- Hardy K. J., Lipton J., Scase M. O., Foster D. H., Scarpello J. H. Detection of colour vision abnormalities in uncomplicated type 1 diabetic patients with angiographically normal retinas. Br J Ophthalmol. 1992 Aug;76(8):461–464. doi: 10.1136/bjo.76.8.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart W. M., Jr Acquired dyschromatopsias. Surv Ophthalmol. 1987 Jul-Aug;32(1):10–31. doi: 10.1016/0039-6257(87)90070-1. [DOI] [PubMed] [Google Scholar]
- Hart W. M., Jr, Becker B. The onset and evolution of glaucomatous visual field defects. Ophthalmology. 1982 Mar;89(3):268–279. doi: 10.1016/s0161-6420(82)34798-3. [DOI] [PubMed] [Google Scholar]
- Hart W. M., Jr, Burde R. M. Three-dimensional topography of the central visual field. Sparing of foveal sensitivity in macular disease. Ophthalmology. 1983 Aug;90(8):1028–1038. doi: 10.1016/s0161-6420(83)80031-1. [DOI] [PubMed] [Google Scholar]
- Hartveit E., Brandstätter J. H., Sassoè-Pognetto M., Laurie D. J., Seeburg P. H., Wässle H. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J Comp Neurol. 1994 Oct 22;348(4):570–582. doi: 10.1002/cne.903480407. [DOI] [PubMed] [Google Scholar]
- Hayreh S. S., Baines J. A. Occlusion of the posterior ciliary artery. I. Effects on choroidal circulation. Br J Ophthalmol. 1972 Oct;56(10):719–735. doi: 10.1136/bjo.56.10.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh S. S., Baines J. A. Occlusion of the posterior ciliary artery. II. Chorio-retinal lesions. Br J Ophthalmol. 1972 Oct;56(10):736–753. doi: 10.1136/bjo.56.10.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh S. S. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969 Nov;53(11):721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh S. S. In vivo choroidal circulation and its watershed zones. Eye (Lond) 1990;4(Pt 2):273–289. doi: 10.1038/eye.1990.39. [DOI] [PubMed] [Google Scholar]
- Hidayat A. A., Fine B. S. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985 Apr;92(4):512–522. [PubMed] [Google Scholar]
- Holopigian K., Greenstein V. C., Seiple W., Hood D. C., Carr R. E. Evidence for photoreceptor changes in patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997 Oct;38(11):2355–2365. [PubMed] [Google Scholar]
- Holopigian K., Seiple W., Mayron C., Koty R., Lorenzo M. Electrophysiological and psychophysical flicker sensitivity in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 1990 Sep;31(9):1863–1868. [PubMed] [Google Scholar]
- Hood D. C., Benimoff N. I., Greenstein V. C. The response range of the blue-cone pathways: a source of vulnerability to disease. Invest Ophthalmol Vis Sci. 1984 Jul;25(7):864–867. [PubMed] [Google Scholar]
- Hood D. C., Frishman L. J., Viswanathan S., Robson J. G., Ahmed J. Evidence for a ganglion cell contribution to the primate electroretinogram (ERG): effects of TTX on the multifocal ERG in macaque. Vis Neurosci. 1999 May-Jun;16(3):411–416. doi: 10.1017/s0952523899163028. [DOI] [PubMed] [Google Scholar]
- Hood D. C., Greenstein V., Frishman L., Holopigian K., Viswanathan S., Seiple W., Ahmed J., Robson J. G. Identifying inner retinal contributions to the human multifocal ERG. Vision Res. 1999 Jun;39(13):2285–2291. doi: 10.1016/s0042-6989(98)00296-x. [DOI] [PubMed] [Google Scholar]
- Janssen P., Naskar R., Moore S., Thanos S., Thiel H. J. Evidence for glaucoma-induced horizontal cell alterations in the human retina. Ger J Ophthalmol. 1996 Nov;5(6):378–385. [PubMed] [Google Scholar]
- Johnson L. V., Hageman G. S. Characterization of molecules bound by the cone photoreceptor-specific monoclonal antibody CSA-1. Invest Ophthalmol Vis Sci. 1988 Apr;29(4):550–557. [PubMed] [Google Scholar]
- Jonas J. B., Gründler A. E. Prevalence of diabetes mellitus and arterial hypertension in primary and secondary open-angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 1998 Mar;236(3):202–206. doi: 10.1007/s004170050065. [DOI] [PubMed] [Google Scholar]
- Kaiser H. J., Flammer J., Stümpfig D., Hendrickson P. Longterm visual field follow-up of glaucoma patients treated with beta-blockers. Surv Ophthalmol. 1994 May;38 (Suppl):S156–S160. doi: 10.1016/0039-6257(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Kaiser H. J., Schoetzau A., Stümpfig D., Flammer J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am J Ophthalmol. 1997 Mar;123(3):320–327. doi: 10.1016/s0002-9394(14)70127-8. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M., Harwerth R. S., Smith E. L., 3rd, DeSantis L. Colour vision anomalies following experimental glaucoma in monkeys. Ophthalmic Physiol Opt. 1993 Jan;13(1):56–67. doi: 10.1111/j.1475-1313.1993.tb00427.x. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. L., Wallow I. H. Minified diagnostic contact lenses for biomicroscopic examination and photocoagulation of the anterior and posterior segment in small primates. Exp Eye Res. 1985 Jun;40(6):883–885. doi: 10.1016/0014-4835(85)90133-2. [DOI] [PubMed] [Google Scholar]
- Kendell K. R., Quigley H. A., Kerrigan L. A., Pease M. E., Quigley E. N. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995 Jan;36(1):200–205. [PubMed] [Google Scholar]
- Kinnear P. R., Aspinall P. A., Lakowski R. The diabetic eye and colour vision. Trans Ophthalmol Soc U K. 1972;92:69–78. [PubMed] [Google Scholar]
- Klein B. E., Klein R., Jensen S. C. Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye Study. Ophthalmology. 1994 Jul;101(7):1173–1177. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- Koliopoulos J., Theodosiadis G. Retinal detachment and acquired colour vision disturbances. Mod Probl Ophthalmol. 1972;11:117–121. [PubMed] [Google Scholar]
- Kroll A. J., Machemer R. Experimental retinal detachment in the owl monkey. 3. Electron microscopy of retina and pigment epithelium. Am J Ophthalmol. 1968 Sep;66(3):410–427. doi: 10.1016/0002-9394(68)91524-9. [DOI] [PubMed] [Google Scholar]
- Kurtenbach A., Schiefer U., Neu A., Zrenner E. Preretinopic changes in the colour vision of juvenile diabetics. Br J Ophthalmol. 1999 Jan;83(1):43–46. doi: 10.1136/bjo.83.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. P., Erickson P. A., Guérin C. J., Anderson D. H., Fisher S. K. Changes in the expression of specific Müller cell proteins during long-term retinal detachment. Exp Eye Res. 1989 Jul;49(1):93–111. doi: 10.1016/0014-4835(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Lewis G. P., Guérin C. J., Anderson D. H., Matsumoto B., Fisher S. K. Rapid changes in the expression of glial cell proteins caused by experimental retinal detachment. Am J Ophthalmol. 1994 Sep 15;118(3):368–376. doi: 10.1016/s0002-9394(14)72962-9. [DOI] [PubMed] [Google Scholar]
- Lutze M., Bresnick G. H. Lenses of diabetic patients "yellow" at an accelerated rate similar to older normals. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):194–199. [PubMed] [Google Scholar]
- Marc R. E., Sperling H. G. Chromatic organization of primate cones. Science. 1977 Apr 22;196(4288):454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Marmor M. F., Kessler R. Sildenafil (Viagra) and ophthalmology. Surv Ophthalmol. 1999 Sep-Oct;44(2):153–162. doi: 10.1016/s0039-6257(99)00079-x. [DOI] [PubMed] [Google Scholar]
- Marmor M. F. Sildenafil (Viagra) and ophthalmology. Arch Ophthalmol. 1999 Apr;117(4):518–519. doi: 10.1001/archopht.117.4.518. [DOI] [PubMed] [Google Scholar]
- McCrane E. P., de Monasterio F. M., Schein S. J., Caruso R. C. Non-fluorescent dye staining of primate blue cones. Invest Ophthalmol Vis Sci. 1983 Nov;24(11):1449–1455. [PubMed] [Google Scholar]
- Michelson G., Groh M. J., Groh M. E., Gründler A. Advanced primary open-angle glaucoma is associated with decreased ophthalmic artery blood-flow velocity. Ger J Ophthalmol. 1995 Jan;4(1):21–24. [PubMed] [Google Scholar]
- Milam A. H., Jacobson S. G. Photoreceptor rosettes with blue cone opsin immunoreactivity in retinitis pigmentosa. Ophthalmology. 1990 Dec;97(12):1620–1631. doi: 10.1016/s0161-6420(90)32358-8. [DOI] [PubMed] [Google Scholar]
- Millar J. C., Wilson W. S., Carr R. D., Humphries R. G. Drug effects on intraocular pressure and vascular flow in the bovine perfused eye using radiolabelled microspheres. J Ocul Pharmacol Ther. 1995 Spring;11(1):11–23. doi: 10.1089/jop.1995.11.11. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Smith W., Chey T., Healey P. R. Open-angle glaucoma and diabetes: the Blue Mountains eye study, Australia. Ophthalmology. 1997 Apr;104(4):712–718. doi: 10.1016/s0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- Moloney J., Drury M. I. Retinopathy and retinal function in insulin-dependent diabetes mellitus. Br J Ophthalmol. 1982 Dec;66(12):759–761. doi: 10.1136/bjo.66.12.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. C., Dorman-Pease M. E., Dunkelberger G. R., Quigley H. A. Optic nerve head extracellular matrix in primary optic atrophy and experimental glaucoma. Arch Ophthalmol. 1990 Jul;108(7):1020–1024. doi: 10.1001/archopht.1990.01070090122053. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi M. Colour vision and dark adaptation in diabetic patients after photocoagulation. Acta Ophthalmol (Copenh) 1989 Apr;67(2):113–118. doi: 10.1111/j.1755-3768.1989.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nickells R. W. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999 Jun;43 (Suppl 1):S151–S161. doi: 10.1016/s0039-6257(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Nork T. M., Mangini N. J., Millecchia L. L. Rods and cones contain antigenically distinctive S-antigens. Invest Ophthalmol Vis Sci. 1993 Sep;34(10):2918–2925. [PubMed] [Google Scholar]
- Nork T. M., McCormick S. A., Chao G. M., Odom J. V. Distribution of carbonic anhydrase among human photoreceptors. Invest Ophthalmol Vis Sci. 1990 Aug;31(8):1451–1458. [PubMed] [Google Scholar]
- Nork T. M., Millecchia L. L., Strickland B. D., Linberg J. V., Chao G. M. Selective loss of blue cones and rods in human retinal detachment. Arch Ophthalmol. 1995 Aug;113(8):1066–1073. doi: 10.1001/archopht.1995.01100080118039. [DOI] [PubMed] [Google Scholar]
- Nork T. M., Ver Hoeve J. N., Poulsen G. L., Nickells R. W., Davis M. D., Weber A. J., Vaegan, Sarks S. H., Lemley H. L., Millecchia L. L. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch Ophthalmol. 2000 Feb;118(2):235–245. doi: 10.1001/archopht.118.2.235. [DOI] [PubMed] [Google Scholar]
- Odom J. V., Feghali J. G., Jin J. C., Weinstein G. W. Visual function deficits in glaucoma. Electroretinogram pattern and luminance nonlinearities. Arch Ophthalmol. 1990 Feb;108(2):222–227. doi: 10.1001/archopht.1990.01070040074034. [DOI] [PubMed] [Google Scholar]
- Ogiso M. [Examination of confusion loci in acquired color vision deficiency with surface color]. Nippon Ganka Gakkai Zasshi. 1993 Mar;97(3):411–418. [PubMed] [Google Scholar]
- Otori Y., Wei J. Y., Barnstable C. J. Neurotoxic effects of low doses of glutamate on purified rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 1998 May;39(6):972–981. [PubMed] [Google Scholar]
- Panda S., Jonas J. B. Decreased photoreceptor count in human eyes with secondary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 1992 Jul;33(8):2532–2536. [PubMed] [Google Scholar]
- Pederson J. E., Gaasterland D. E. Laser-induced primate glaucoma. I. Progression of cupping. Arch Ophthalmol. 1984 Nov;102(11):1689–1692. doi: 10.1001/archopht.1984.01040031373030. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Kiland J. A., Croft M. A., Kaufman P. L. Intraocular pressure measurement in cynomolgus monkeys. Tono-Pen versus manometry. Invest Ophthalmol Vis Sci. 1996 May;37(6):1197–1199. [PubMed] [Google Scholar]
- Poinoosawmy D., Nagasubramanian S., Gloster J. Colour vision in patients with chronic simple glaucoma and ocular hypertension. Br J Ophthalmol. 1980 Nov;64(11):852–857. doi: 10.1136/bjo.64.11.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H. A., Addicks E. M., Green W. R., Maumenee A. E. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981 Apr;99(4):635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- Quigley H. A., Addicks E. M., Green W. R. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982 Jan;100(1):135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- Quigley H. A., Addicks E. M. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981 Jan;99(1):137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- Quigley H. A., Dunkelberger G. R., Green W. R. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988 Mar;95(3):357–363. doi: 10.1016/s0161-6420(88)33176-3. [DOI] [PubMed] [Google Scholar]
- Quigley H. A., Green W. R. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979 Oct;86(10):1803–1830. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- Quigley H. A., Hohman R. M. Laser energy levels for trabecular meshwork damage in the primate eye. Invest Ophthalmol Vis Sci. 1983 Sep;24(9):1305–1307. [PubMed] [Google Scholar]
- Quigley H. A., Nickells R. W., Kerrigan L. A., Pease M. E., Thibault D. J., Zack D. J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995 Apr;36(5):774–786. [PubMed] [Google Scholar]
- Quigley H. A., Sanchez R. M., Dunkelberger G. R., L'Hernault N. L., Baginski T. A. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):913–920. [PubMed] [Google Scholar]
- Ridderstråle Y. Intracellular localization of carbonic anhydrase in the frog nephron. Acta Physiol Scand. 1976 Dec;98(4):465–469. doi: 10.1111/j.1748-1716.1976.tb10337.x. [DOI] [PubMed] [Google Scholar]
- Rockett M., Anderle D., Bessman A. N. Blue-yellow vision deficits in patients with diabetes. West J Med. 1987 Apr;146(4):431–433. [PMC free article] [PubMed] [Google Scholar]
- Rojanapongpun P., Drance S. M., Morrison B. J. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993 Jan;77(1):25–29. doi: 10.1136/bjo.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roorda A., Williams D. R. The arrangement of the three cone classes in the living human eye. Nature. 1999 Feb 11;397(6719):520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- Rosen E. S., Boyd T. A. New method of assessing choroidal ischemia in open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1970 Dec;70(6):912–921. doi: 10.1016/0002-9394(70)92467-0. [DOI] [PubMed] [Google Scholar]
- Roy M. S., Gunkel R. D., Podgor M. J. Color vision defects in early diabetic retinopathy. Arch Ophthalmol. 1986 Feb;104(2):225–228. doi: 10.1001/archopht.1986.01050140079024. [DOI] [PubMed] [Google Scholar]
- Röhlich P., Szél A., Johnson L. V., Hageman G. S. Carbohydrate components recognized by the cone-specific monoclonal antibody CSA-1 and by peanut agglutinin are associated with red and green-sensitive cone photoreceptors. J Comp Neurol. 1989 Nov 15;289(3):395–400. doi: 10.1002/cne.902890305. [DOI] [PubMed] [Google Scholar]
- Röhrenbeck Jürgen, Wässle Heinz, Boycott Brian B. Horizontal Cells in the Monkey Retina: Immunocytochemical staining with antibodies against calcium binding proteins. Eur J Neurosci. 1989 Sep;1(5):407–420. doi: 10.1111/j.1460-9568.1989.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Seiple W. H., Holopigian K., Greenstein V. C., Hood D. C. Sites of cone system sensitivity loss in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1993 Aug;34(9):2638–2645. [PubMed] [Google Scholar]
- Siliprandi R., Canella R., Carmignoto G., Schiavo N., Zanellato A., Zanoni R., Vantini G. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992 Jun;8(6):567–573. doi: 10.1017/s0952523800005666. [DOI] [PubMed] [Google Scholar]
- Silverman S. E., Hart W. M., Jr, Gordon M. O., Kilo C. The dyschromatopsia of optic neuritis is determined in part by the foveal/perifoveal distribution of visual field damage. Invest Ophthalmol Vis Sci. 1990 Sep;31(9):1895–1902. [PubMed] [Google Scholar]
- Sperling H. G., Johnson C., Harwerth R. S. Differential spectral photic damage to primate cones. Vision Res. 1980;20(12):1117–1125. doi: 10.1016/0042-6989(80)90049-8. [DOI] [PubMed] [Google Scholar]
- Stern W. H., Ernest J. T. Microsphere occlusion of the choriocapillaris in rhesus monkeys. Am J Ophthalmol. 1974 Sep;78(3):438–448. doi: 10.1016/0002-9394(74)90231-1. [DOI] [PubMed] [Google Scholar]
- Stone J. L., Barlow W. E., Humayun M. S., de Juan E., Jr, Milam A. H. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992 Nov;110(11):1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Birch D. G., Anderson J. L. S-cone function in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1993 Oct;34(11):3045–3055. [PubMed] [Google Scholar]
- Szél A., Takács L., Monostori E., Diamantstein T., Vigh-Teichmann I., Röhlich P. Monoclonal antibody-recognizing cone visual pigment. Exp Eye Res. 1986 Dec;43(6):871–883. doi: 10.1016/0014-4835(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Terasaki H., Hirose H., Miyake Y. S-cone pathway sensitivity in diabetes measured with threshold versus intensity curves on flashed backgrounds. Invest Ophthalmol Vis Sci. 1996 Mar;37(4):680–684. [PubMed] [Google Scholar]
- Tielsch J. M., Katz J., Singh K., Quigley H. A., Gottsch J. D., Javitt J., Sommer A. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991 Nov 15;134(10):1102–1110. doi: 10.1093/oxfordjournals.aje.a116013. [DOI] [PubMed] [Google Scholar]
- Tregear S. J., Knowles P. J., Ripley L. G., Casswell A. G. Chromatic-contrast threshold impairment in diabetes. Eye (Lond) 1997;11(Pt 4):537–546. doi: 10.1038/eye.1997.140. [DOI] [PubMed] [Google Scholar]
- Vaegan, Buckland L. The spatial distribution of ERG losses across the posterior pole of glaucomatous eyes in multifocal recordings. Aust N Z J Ophthalmol. 1996 May;24(2 Suppl):28–31. doi: 10.1111/j.1442-9071.1996.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Vaegan, Graham S. L., Goldberg I., Buckland L., Hollows F. C. Flash and pattern electroretinogram changes with optic atrophy and glaucoma. Exp Eye Res. 1995 Jun;60(6):697–706. doi: 10.1016/s0014-4835(05)80011-9. [DOI] [PubMed] [Google Scholar]
- Vainio-Jylhä E., Vuori M. L. The favorable effect of topical betaxolol and timolol on glaucomatous visual fields: a 2-year follow-up study. Graefes Arch Clin Exp Ophthalmol. 1999 Feb;237(2):100–104. doi: 10.1007/s004170050202. [DOI] [PubMed] [Google Scholar]
- Vorwerk C. K., Lipton S. A., Zurakowski D., Hyman B. T., Sabel B. A., Dreyer E. B. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996 Jul;37(8):1618–1624. [PubMed] [Google Scholar]
- Wallow I. H. Repair of the pigment epithelial barrier following photocoagulation. Arch Ophthalmol. 1984 Jan;102(1):126–135. doi: 10.1001/archopht.1984.01040030104047. [DOI] [PubMed] [Google Scholar]
- Wallow I. H., Sponsel W. E., Stevens T. S. Clinicopathologic correlation of diode laser burns in monkeys. Arch Ophthalmol. 1991 May;109(5):648–653. doi: 10.1001/archopht.1991.01080050062030. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Mishina M., Inoue Y. Differential distributions of the NMDA receptor channel subunit mRNAs in the mouse retina. Brain Res. 1994 Jan 21;634(2):328–332. doi: 10.1016/0006-8993(94)91938-0. [DOI] [PubMed] [Google Scholar]
- Weiner A., Ripkin D. J., Patel S., Kaufman S. R., Kohn H. D., Weidenthal D. T. Foveal dysfunction and central visual field loss in glaucoma. Arch Ophthalmol. 1998 Sep;116(9):1169–1174. doi: 10.1001/archopht.116.9.1169. [DOI] [PubMed] [Google Scholar]
- Wygnanski T., Desatnik H., Quigley H. A., Glovinsky Y. Comparison of ganglion cell loss and cone loss in experimental glaucoma. Am J Ophthalmol. 1995 Aug;120(2):184–189. doi: 10.1016/s0002-9394(14)72606-6. [DOI] [PubMed] [Google Scholar]
- Xiao M., Sastry S. M., Li Z. Y., Possin D. E., Chang J. H., Klock I. B., Milam A. H. Effects of retinal laser photocoagulation on photoreceptor basic fibroblast growth factor and survival. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):618–630. [PubMed] [Google Scholar]
- Yamamoto R., Bredt D. S., Dawson T. M., Snyder S. H., Stone R. A. Enhanced expression of nitric oxide synthase by rat retina following pterygopalatine parasympathetic denervation. Brain Res. 1993 Dec 17;631(1):83–88. doi: 10.1016/0006-8993(93)91190-4. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Drance S. M. The relationship between progression of visual field defects and retrobulbar circulation in patients with glaucoma. Am J Ophthalmol. 1997 Sep;124(3):287–295. doi: 10.1016/s0002-9394(14)70820-7. [DOI] [PubMed] [Google Scholar]
- Yancey C. M., Linsenmeier R. A. Oxygen distribution and consumption in the cat retina at increased intraocular pressure. Invest Ophthalmol Vis Sci. 1989 Apr;30(4):600–611. [PubMed] [Google Scholar]
- Yancey C. M., Linsenmeier R. A. The electroretinogram and choroidal PO2 in the cat during elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1988 May;29(5):700–707. [PubMed] [Google Scholar]
- Yin Z. Q., Vaegan, Millar T. J., Beaumont P., Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997 Feb;6(1):23–32. [PubMed] [Google Scholar]
- Yoles E., Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998 Jul;116(7):906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- Yoneya S., Tso M. O. Angioarchitecture of the human choroid. Arch Ophthalmol. 1987 May;105(5):681–687. doi: 10.1001/archopht.1987.01060050099046. [DOI] [PubMed] [Google Scholar]
- Zrenner E., Gouras P. Blue-sensitive cones of the cat produce a rodlike electroretinogram. Invest Ophthalmol Vis Sci. 1979 Oct;18(10):1076–1081. [PubMed] [Google Scholar]
- de Monasterio F. M. Asymmetry of on- and off-pathways of blue-sensitive cones of the retina of macaques. Brain Res. 1979 Apr 20;166(1):39–48. doi: 10.1016/0006-8993(79)90647-4. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M., McCrane E. P., Newlander J. K., Schein S. J. Density profile of blue-sensitive cones along the horizontal meridian of macaque retina. Invest Ophthalmol Vis Sci. 1985 Mar;26(3):289–302. [PubMed] [Google Scholar]