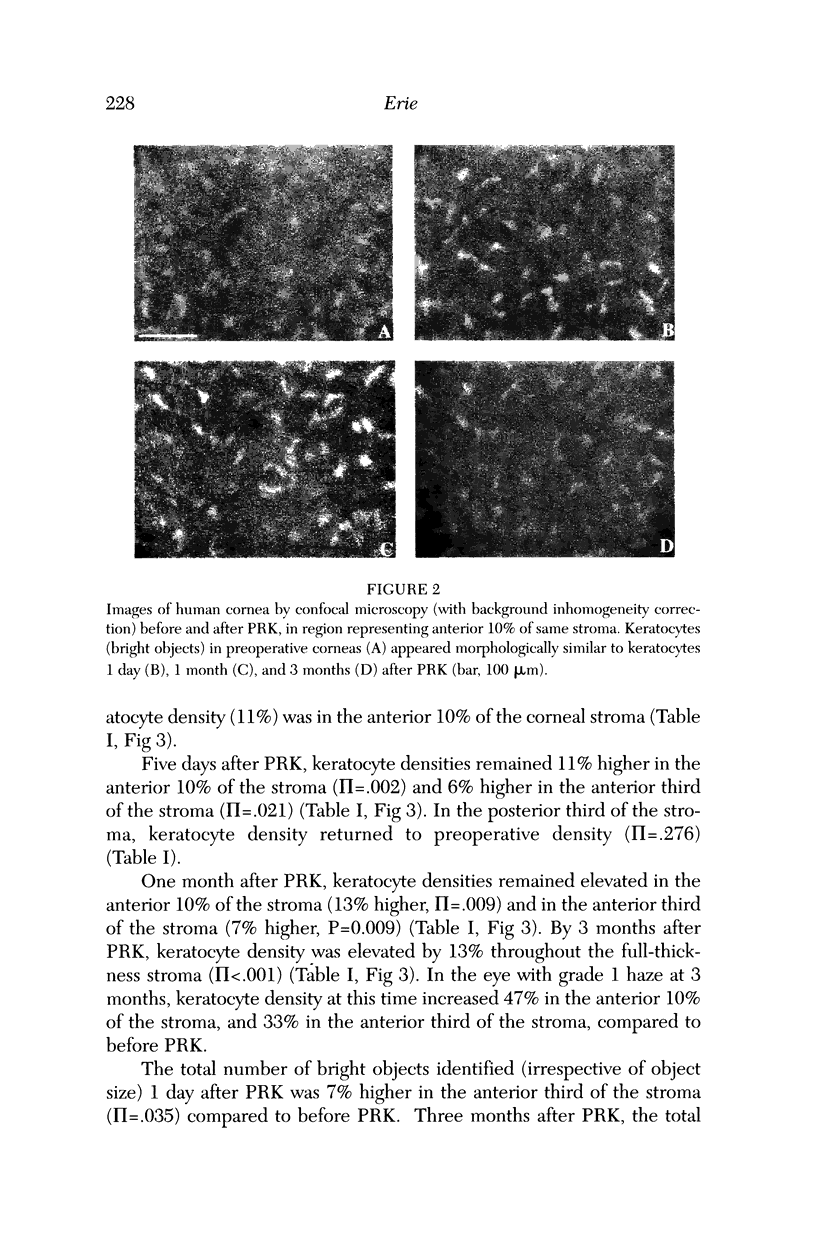
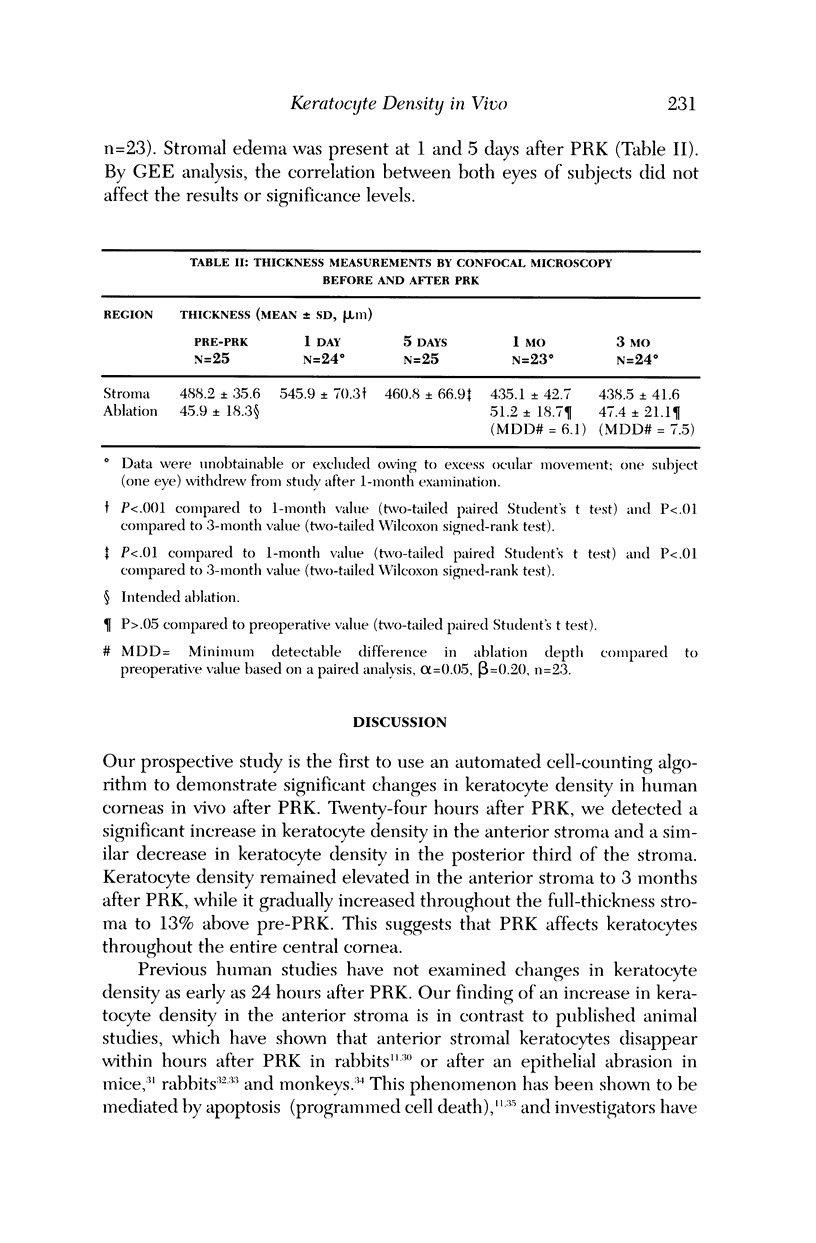
Abstract
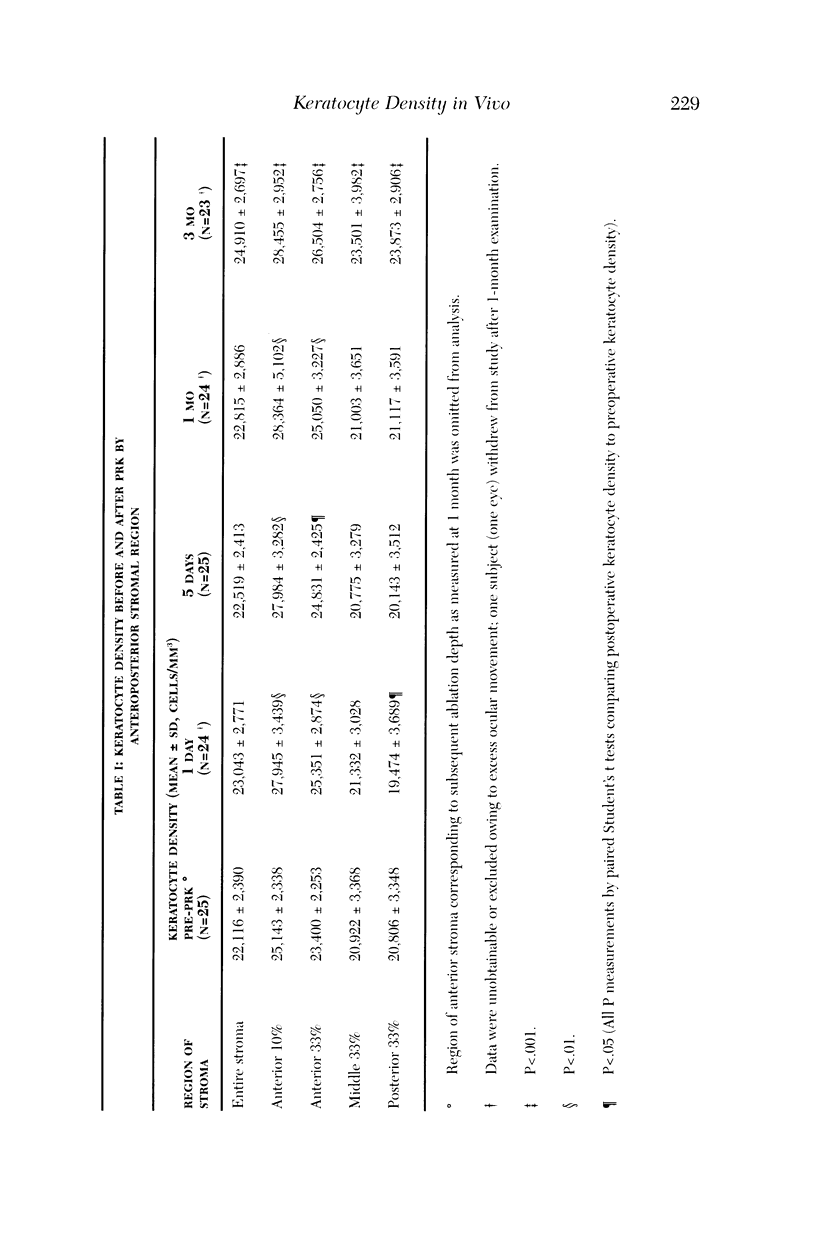
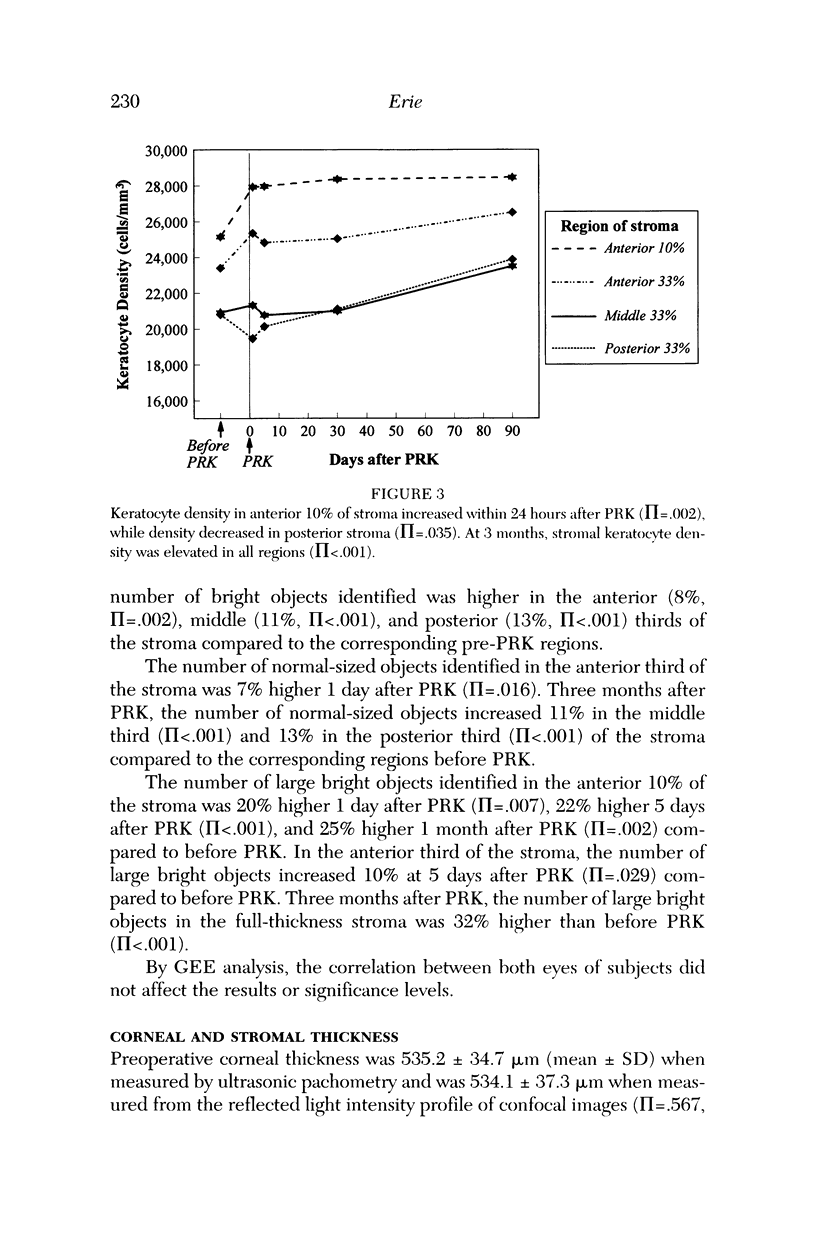
PURPOSE: To determine changes in keratocyte density in central human corneas in vivo after photorefractive keratectomy (PRK). METHODS: Fifteen patients (25 eyes) received excimer PRK (VISX Star) with epithelial removal by laser-scrape (43 microns ablation followed by manual scrape) to correct myopia between -1.5 D and -7.25 D. Corneas were examined by using confocal microscopy in vivo before PRK and at 1 day, 5 days, 1 month, and 3 months after PRK. A custom automated image-processing algorithm identified bright objects (keratocytes) against a dark background and estimated keratocyte density by using the number and size of the objects. Cell density was quantified in anteroposterior stromal regions after PRK and compared to cell density in corresponding pre-PRK regions. RESULTS: One day after PRK, keratocyte density increased 9% in the anterior third of the stroma (pi = .003), was unchanged in the middle third of the stroma (pi = .481), and decreased 6% in the posterior third of the stroma (pi = .038). Keratocyte density remained elevated in the anterior stroma to 3 months after PRK; at this time, there was a 13% increase in keratocyte density throughout the full-thickness stroma (pi < .001). CONCLUSIONS: Keratocyte density was increased in the anterior stroma immediately after PRK in humans. Three months later, keratocyte density was increased in all anteroposterior stromal regions, suggesting that PRK affects keratocytes throughout the entire central cornea.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balestrazzi E., De Molfetta V., Spadea L., Vinciguerra P., Palmieri G., Santeusanio G., Spagnoli L. Histological, immunohistochemical, and ultrastructural findings in human corneas after photorefractive keratectomy. J Refract Surg. 1995 May-Jun;11(3):181–187. [PubMed] [Google Scholar]
- Beuerman R. W., McDonald M. B., Shofner R. S., Munnerlyn C. R., Clapham T. N., Salmeron B., Kaufman H. E. Quantitative histological studies of primate corneas after excimer laser photorefractive keratectomy. Arch Ophthalmol. 1994 Aug;112(8):1103–1110. doi: 10.1001/archopht.1994.01090200109031. [DOI] [PubMed] [Google Scholar]
- Brennan N. A., Efron N., Carney L. G. Corneal oxygen availability during contact lens wear: a comparison of methodologies. Am J Optom Physiol Opt. 1988 Jan;65(1):19–24. doi: 10.1097/00006324-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Campos M., Raman S., Lee M., McDonnell P. J. Keratocyte loss after different methods of de-epithelialization. Ophthalmology. 1994 May;101(5):890–894. doi: 10.1016/s0161-6420(94)31242-5. [DOI] [PubMed] [Google Scholar]
- Cavanagh H. D., Petroll W. M., Alizadeh H., He Y. G., McCulley J. P., Jester J. V. Clinical and diagnostic use of in vivo confocal microscopy in patients with corneal disease. Ophthalmology. 1993 Oct;100(10):1444–1454. doi: 10.1016/s0161-6420(93)31457-0. [DOI] [PubMed] [Google Scholar]
- Corbett M. C., Prydal J. I., Verma S., Oliver K. M., Pande M., Marshall J. An in vivo investigation of the structures responsible for corneal haze after photorefractive keratectomy and their effect on visual function. Ophthalmology. 1996 Sep;103(9):1366–1380. doi: 10.1016/s0161-6420(96)30495-8. [DOI] [PubMed] [Google Scholar]
- Del Pero R. A., Gigstad J. E., Roberts A. D., Klintworth G. K., Martin C. A., L'Esperance F. A., Jr, Taylor D. M. A refractive and histopathologic study of excimer laser keratectomy in primates. Am J Ophthalmol. 1990 Apr 15;109(4):419–429. doi: 10.1016/s0002-9394(14)74608-2. [DOI] [PubMed] [Google Scholar]
- Essepian J. P., Rajpal R. K., Azar D. T., New K., Antonacci R., Shields W., Stark W. J. The use of confocal microscopy in evaluating corneal wound healing after excimer laser keratectomy. Scanning. 1994 Sep-Oct;16(5):300–304. doi: 10.1002/sca.4950160508. [DOI] [PubMed] [Google Scholar]
- Fantes F. E., Hanna K. D., Waring G. O., 3rd, Pouliquen Y., Thompson K. P., Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990 May;108(5):665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- Frueh B. E., Cadez R., Böhnke M. In vivo confocal microscopy after photorefractive keratectomy in humans. A prospective, long-term study. Arch Ophthalmol. 1998 Nov;116(11):1425–1431. doi: 10.1001/archopht.116.11.1425. [DOI] [PubMed] [Google Scholar]
- Hanna K. D., Pouliquen Y., Waring G. O., 3rd, Savoldelli M., Cotter J., Morton K., Menasche M. Corneal stromal wound healing in rabbits after 193-nm excimer laser surface ablation. Arch Ophthalmol. 1989 Jun;107(6):895–901. doi: 10.1001/archopht.1989.01070010917041. [DOI] [PubMed] [Google Scholar]
- Hanson D. P., Robb R. A., Aharon S., Augustine K. E., Cameron B. M., Camp J. J., Karwoski R. A., Larson A. G., Stacy M. C., Workman E. L. New software toolkits for comprehensive visualization and analysis of three-dimensional multimodal biomedical images. J Digit Imaging. 1997 Aug;10(3 Suppl 1):229–230. doi: 10.1007/BF03168711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helena M. C., Baerveldt F., Kim W. J., Wilson S. E. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):276–283. [PubMed] [Google Scholar]
- Helena M. C., Baerveldt F., Kim W. J., Wilson S. E. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):276–283. [PubMed] [Google Scholar]
- Jester J. V., Petroll W. M., Barry P. A., Cavanagh H. D. Temporal, 3-dimensional, cellular anatomy of corneal wound tissue. J Anat. 1995 Apr;186(Pt 2):301–311. [PMC free article] [PubMed] [Google Scholar]
- Jester J. V., Petroll W. M., Cavanagh H. D. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999 May;18(3):311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jester J. V., Petroll W. M., Garana R. M., Lemp M. A., Cavanagh H. D. Comparison of in vivo and ex vivo cellular structure in rabbit eyes detected by tandem scanning microscopy. J Microsc. 1992 Jan;165(Pt 1):169–181. doi: 10.1111/j.1365-2818.1992.tb04314.x. [DOI] [PubMed] [Google Scholar]
- Kratz-Owens K. L., Hageman G. S., Schanzlin D. J. An in-vivo technique for monitoring keratocyte migration following lamellar keratoplasty. Refract Corneal Surg. 1992 May-Jun;8(3):230–234. [PubMed] [Google Scholar]
- Li H. F., Petroll W. M., Møller-Pedersen T., Maurer J. K., Cavanagh H. D., Jester J. V. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF). Curr Eye Res. 1997 Mar;16(3):214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- Linna T., Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997 Jul;16(7):640–649. doi: 10.1076/ceyr.16.7.640.5058. [DOI] [PubMed] [Google Scholar]
- Møller-Pedersen T., Cavanagh H. D., Petroll W. M., Jester J. V. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998 Nov;17(6):627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Møller-Pedersen T., Li H. F., Petroll W. M., Cavanagh H. D., Jester J. V. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):487–501. [PubMed] [Google Scholar]
- Møller-Pedersen T., Li H. F., Petroll W. M., Cavanagh H. D., Jester J. V. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998 Mar;39(3):487–501. [PubMed] [Google Scholar]
- Møller-Pedersen T., Vogel M., Li H. F., Petroll W. M., Cavanagh H. D., Jester J. V. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997 Mar;104(3):360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- Müller L. J., Pels L., Vrensen G. F. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996 Mar;37(4):476–488. [PubMed] [Google Scholar]
- Patel S. V., McLaren J. W., Camp J. J., Nelson L. R., Bourne W. M. Automated quantification of keratocyte density by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 1999 Feb;40(2):320–326. [PubMed] [Google Scholar]
- Patel S. V., McLaren J. W., Camp J. J., Nelson L. R., Bourne W. M. Automated quantification of keratocyte density by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 1999 Feb;40(2):320–326. [PubMed] [Google Scholar]
- Seiler T., Holschbach A., Derse M., Jean B., Genth U. Complications of myopic photorefractive keratectomy with the excimer laser. Ophthalmology. 1994 Jan;101(1):153–160. doi: 10.1016/s0161-6420(94)31371-6. [DOI] [PubMed] [Google Scholar]
- Szerenyi K. D., Wang X., Gabrielian K., McDonnell P. J. Keratocyte loss and repopulation of anterior corneal stroma after de-epithelialization. Arch Ophthalmol. 1994 Jul;112(7):973–976. doi: 10.1001/archopht.1994.01090190121031. [DOI] [PubMed] [Google Scholar]
- Taylor D. M., L'Esperance F. A., Jr, Del Pero R. A., Roberts A. D., Gigstad J. E., Klintworth G., Martin C. A., Warner J. Human excimer laser lamellar keratectomy. A clinical study. Ophthalmology. 1989 May;96(5):654–664. doi: 10.1016/s0161-6420(89)32836-3. [DOI] [PubMed] [Google Scholar]
- Tuft S. J., Gartry D. S., Rawe I. M., Meek K. M. Photorefractive keratectomy: implications of corneal wound healing. Br J Ophthalmol. 1993 Apr;77(4):243–247. doi: 10.1136/bjo.77.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. E., He Y. G., Weng J., Li Q., McDowall A. W., Vital M., Chwang E. L. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996 Apr;62(4):325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson S. E., Liu J. J., Mohan R. R. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999 May;18(3):293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Stark W. J., Green W. R. Corneal wound healing after 193-nm excimer laser keratectomy. Arch Ophthalmol. 1991 Oct;109(10):1426–1432. doi: 10.1001/archopht.1991.01080100106053. [DOI] [PubMed] [Google Scholar]