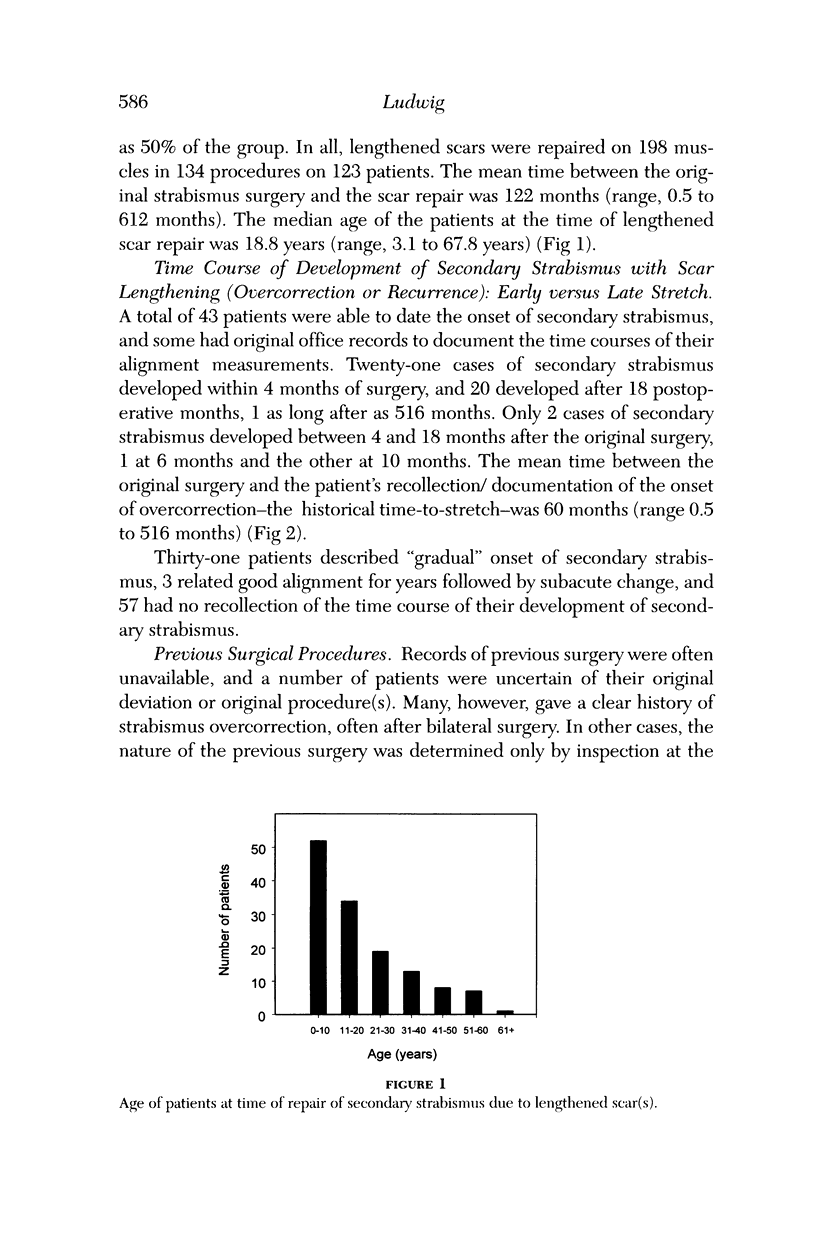
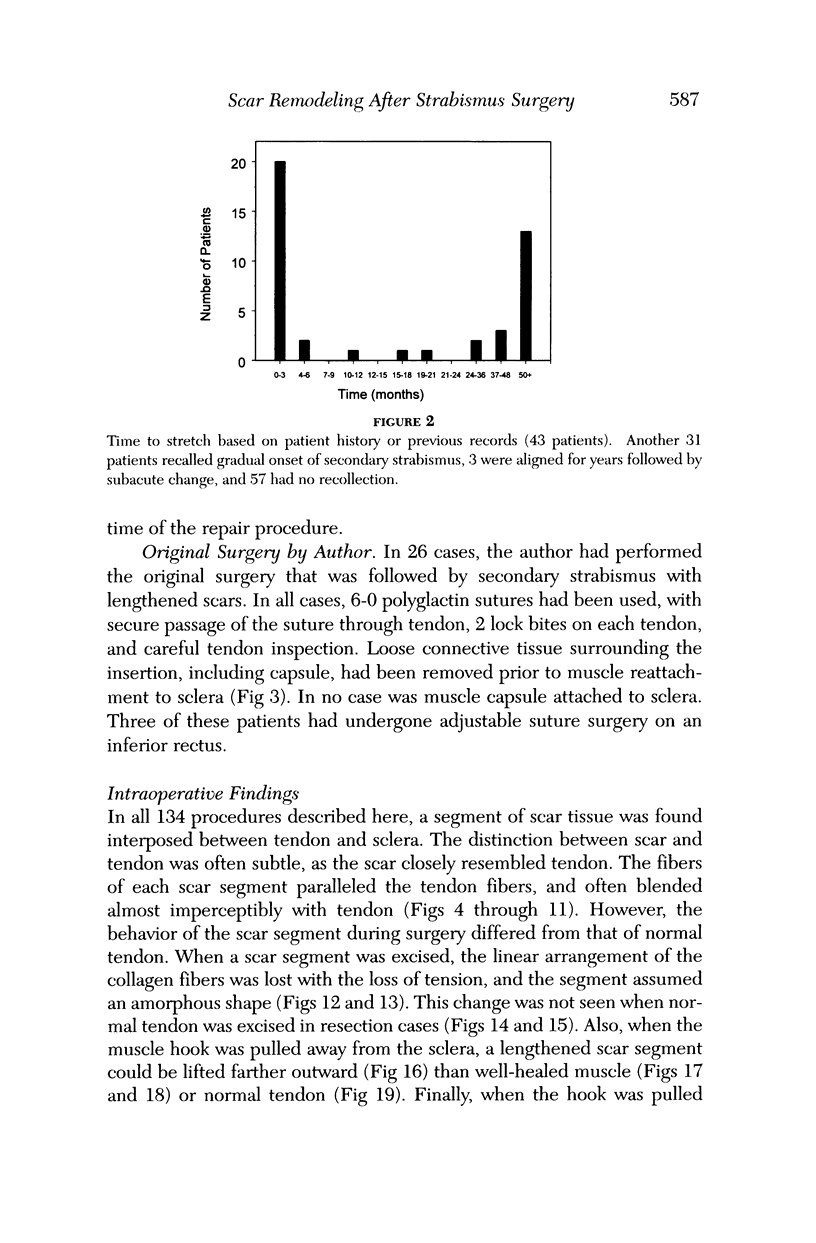
Abstract
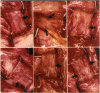
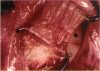
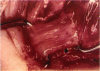
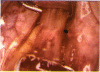
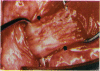
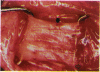
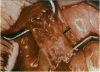
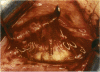
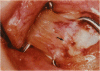
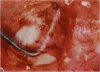
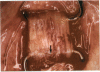
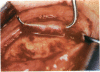
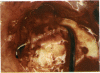
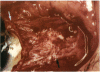
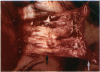
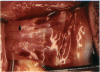
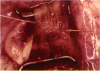
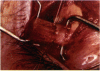
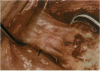
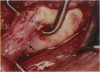
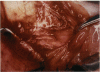
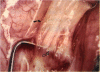
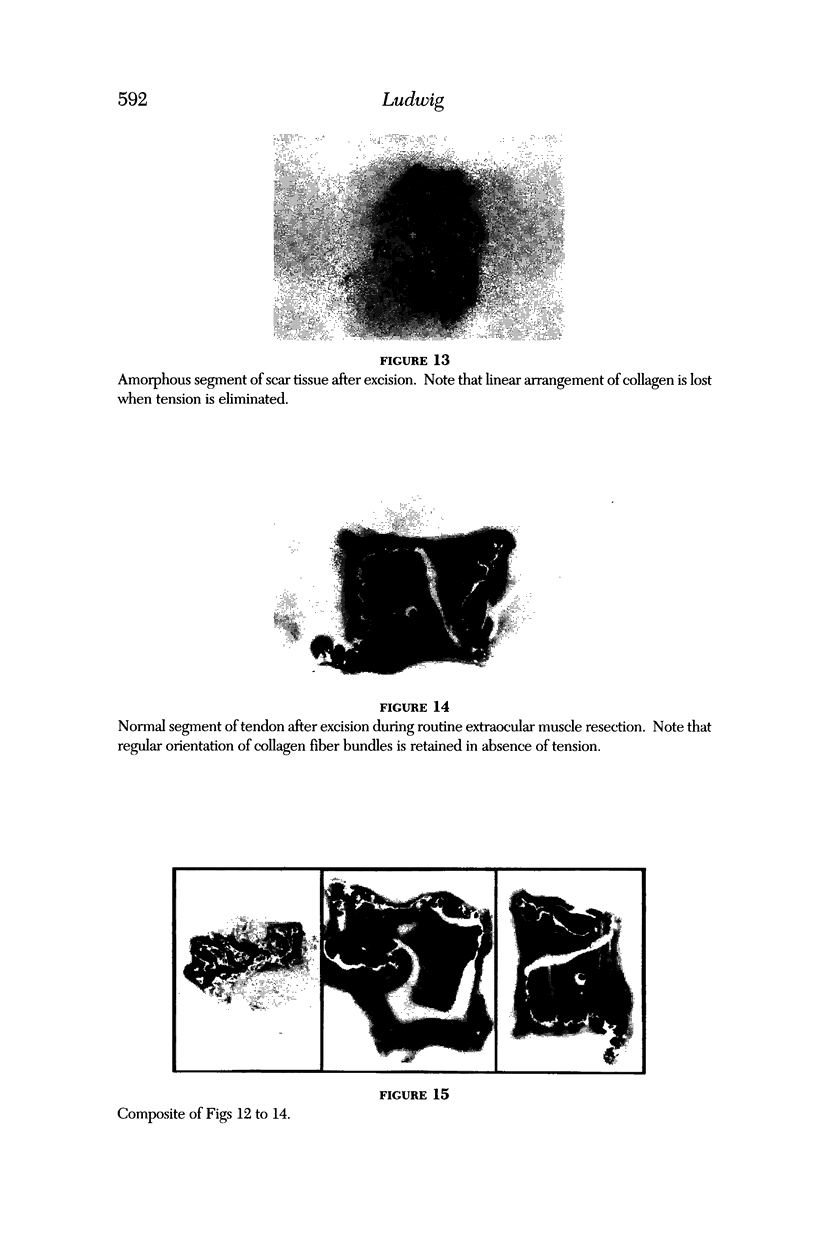
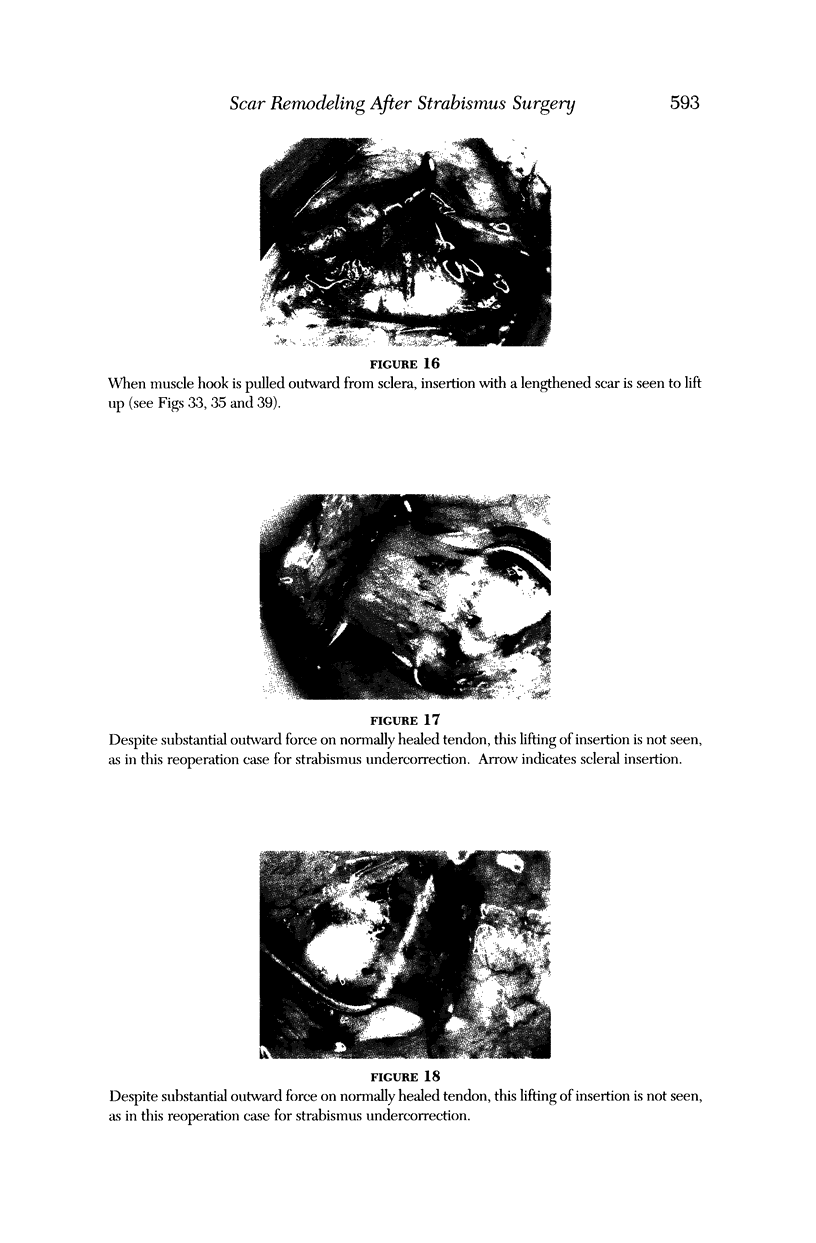
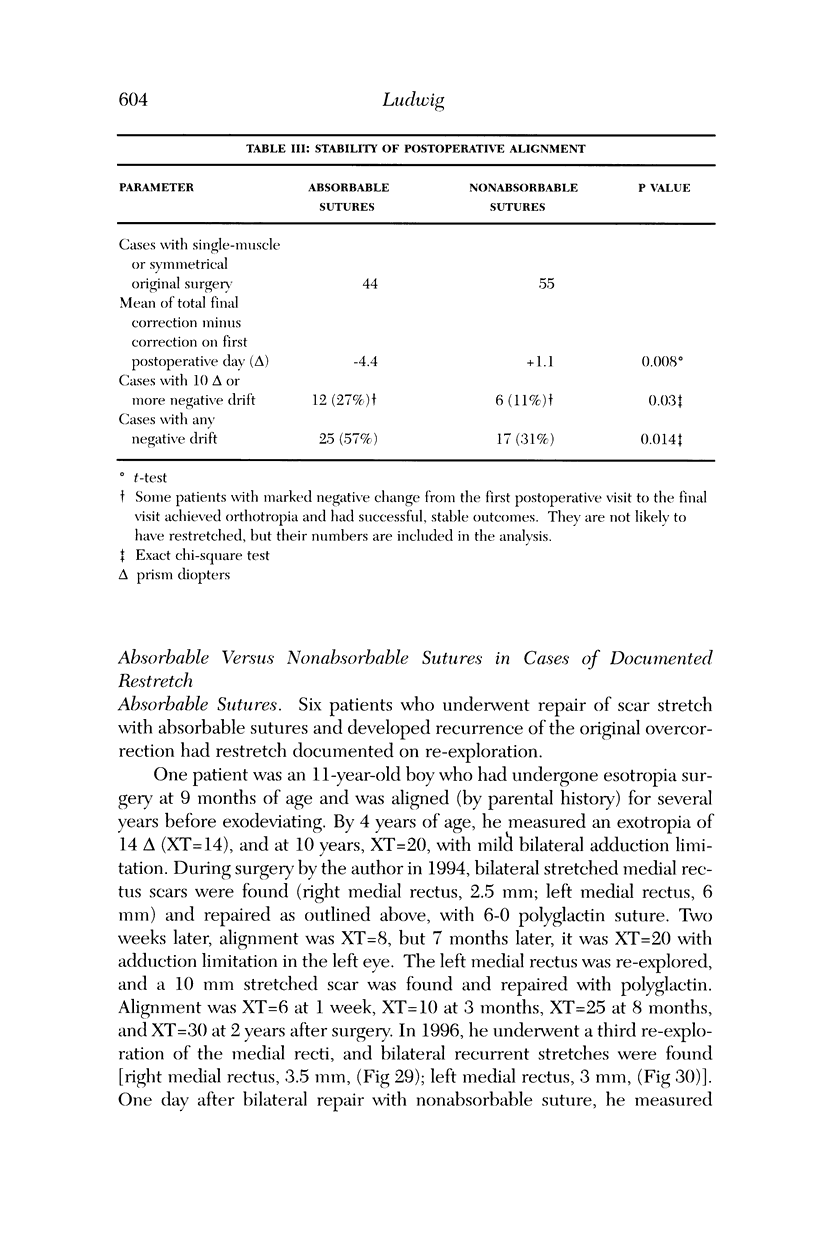
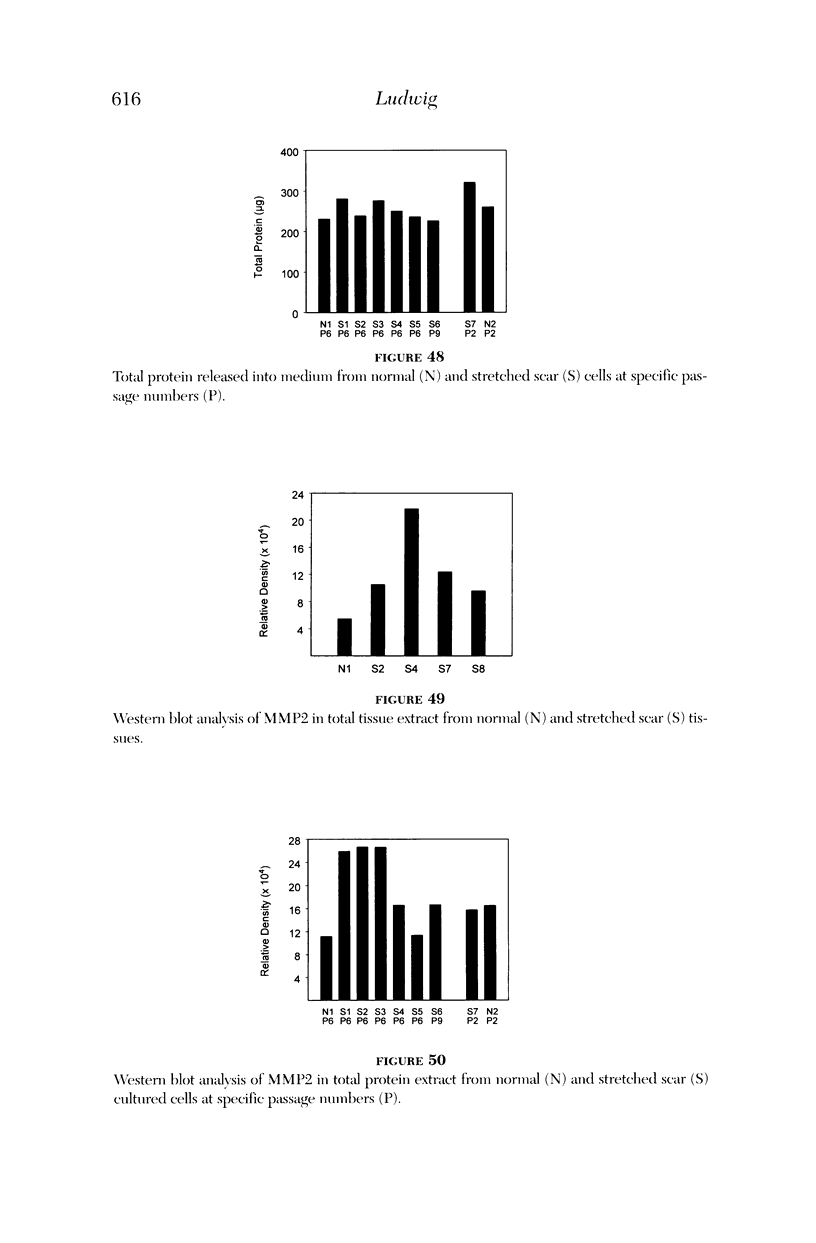
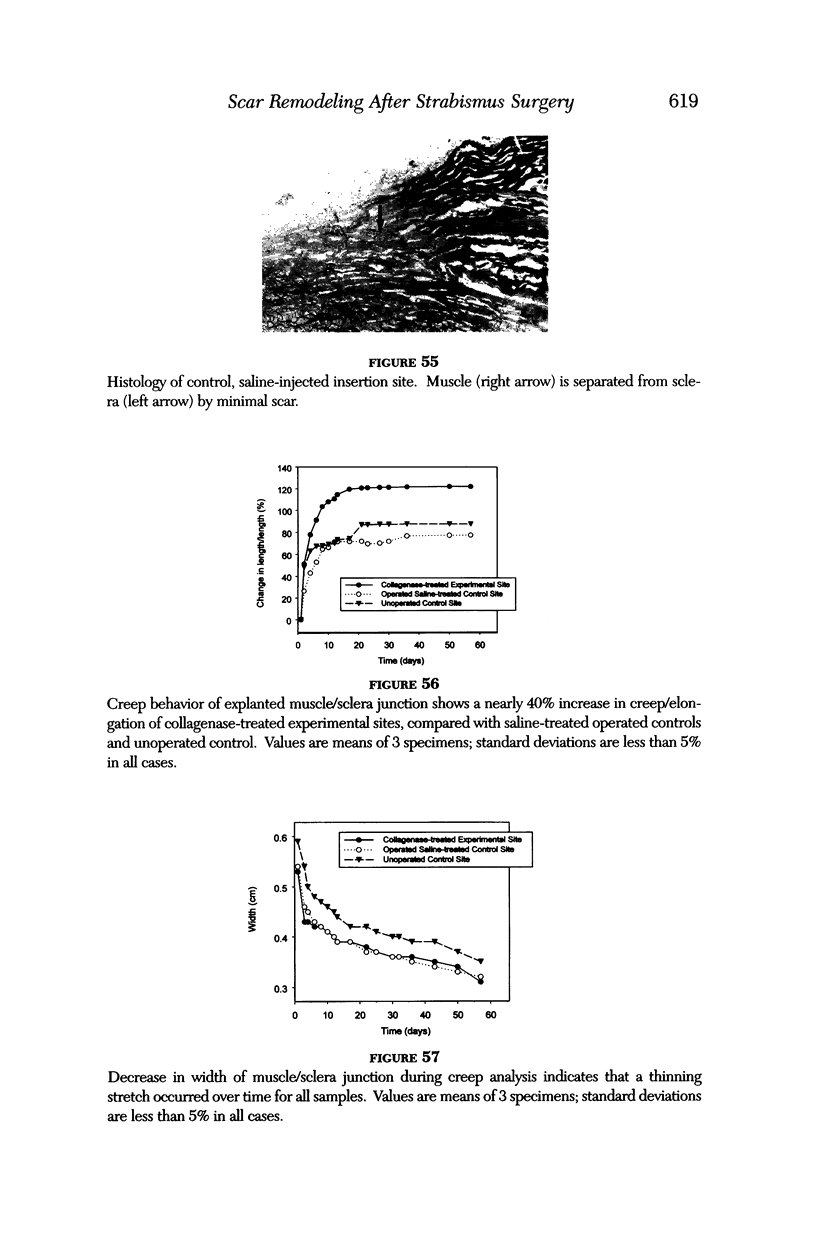
PURPOSE: Patients with overcorrected strabismus (and several patients with undercorrection after extraocular muscle resection) underwent exploration of previously operated muscles, with the intention of advancing their tendons to prevent the need for surgery on additional muscles. Unexpectedly, it was found that, in many cases, an elongated scar segment of variable length was interposed between the muscle and its insertion site on the sclera. Laboratory investigations were carried out to elucidate the underlying mechanism(s) and to create an animal model of the disorder. METHODS: Lengthened scars were repaired on 198 muscles during 134 procedures performed on 123 patients. The scars consisted of amorphous connective tissue interposed between the globe and normal tendon. Repair was accomplished by excision of the scar and reattachment of the muscle to sclera, using absorbable sutures in 64 cases and nonabsorbable sutures in 70 cases. Histopathologic examination was performed on 82 clinical specimens, and tissue culture studies were performed on 7 specimens. To develop an animal model, 10 New Zealand white rabbits underwent bilateral superior rectus resection. Half of the eyes received sub-Tenon's injections of collagenase over the operative site during weeks 2, 3, 5, and 6 postoperatively; the other half received saline solution injections on the same schedule. At 10 weeks, half the sites were studied histologically, and the other half underwent collagen creep analysis. In a second study, the use of absorbable versus nonabsorbable sutures was compared in the rabbit model. RESULTS: In the clinical cases, the mean length of the elongated scar segments was 4.2 mm. A total of 105 of the 134 repair procedures were judged successful. Thirty-one procedures resulted in recurrence of the original overcorrection; 7 of these had documented restretches. Factors that distinguished patients with stretched scars from patients with classic slipped muscles included minimal or no limitation of versions, less separation of the tendons from sclera, and thicker appearance of the scar segments. The use of nonabsorbable sutures in the repair procedure reduced the recurrence rate. Histologic examination of the clinical stretched scar specimens showed dense connective tissue that was less well organized compared with normal tendon. In the tissue culture studies, cells cultured from the stretched scar specimens grew rapidly and were irregularly shaped. A high-molecular-weight protein was identified in the culture medium. By contrast, cells cultured from normal tendon (controls) grew more slowly and regularly, stopped growing at 4 days, and produced less total protein than cultured stretched scar specimens. In the animal model studies, the collagenase-treated sites showed elongated scars with increased collagen between the muscle and the sclera, as well as increased collagen creep rates, compared with the saline-treated controls. The use of nonabsorbable sutures in collagenase-treated animal model surgery sites was associated with shorter, thicker scars compared with similar sites sutured with absorbable sutures. CONCLUSIONS: A lengthened or stretched, remodeled scar between an operated muscle tendon and sclera is a common occurrence and is a factor contributing to the variability of outcome after strabismus repair, even years later. This abnormality may be revealed by careful exploration of previously operated muscles. Definitive repair requires firm reattachment of tendon to sclera with nonabsorbable suture support.
Full text
PDF
























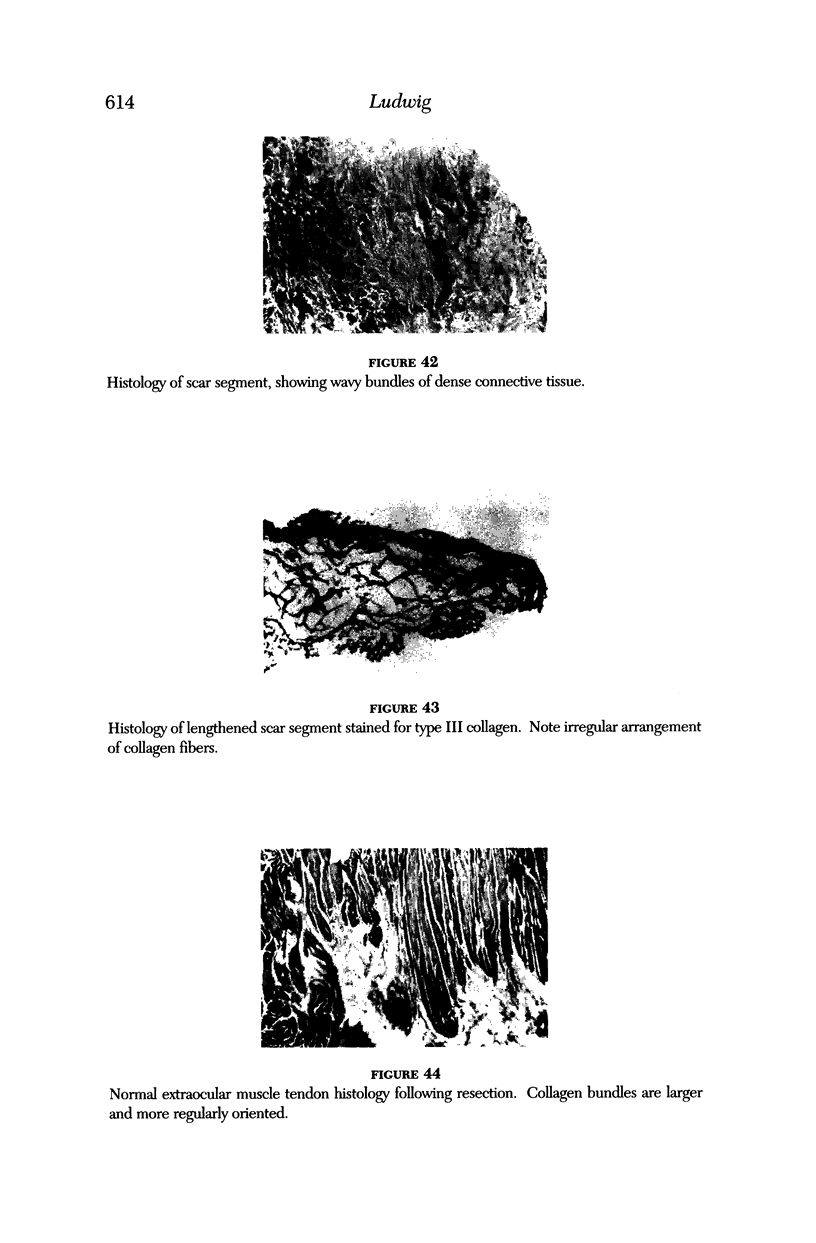
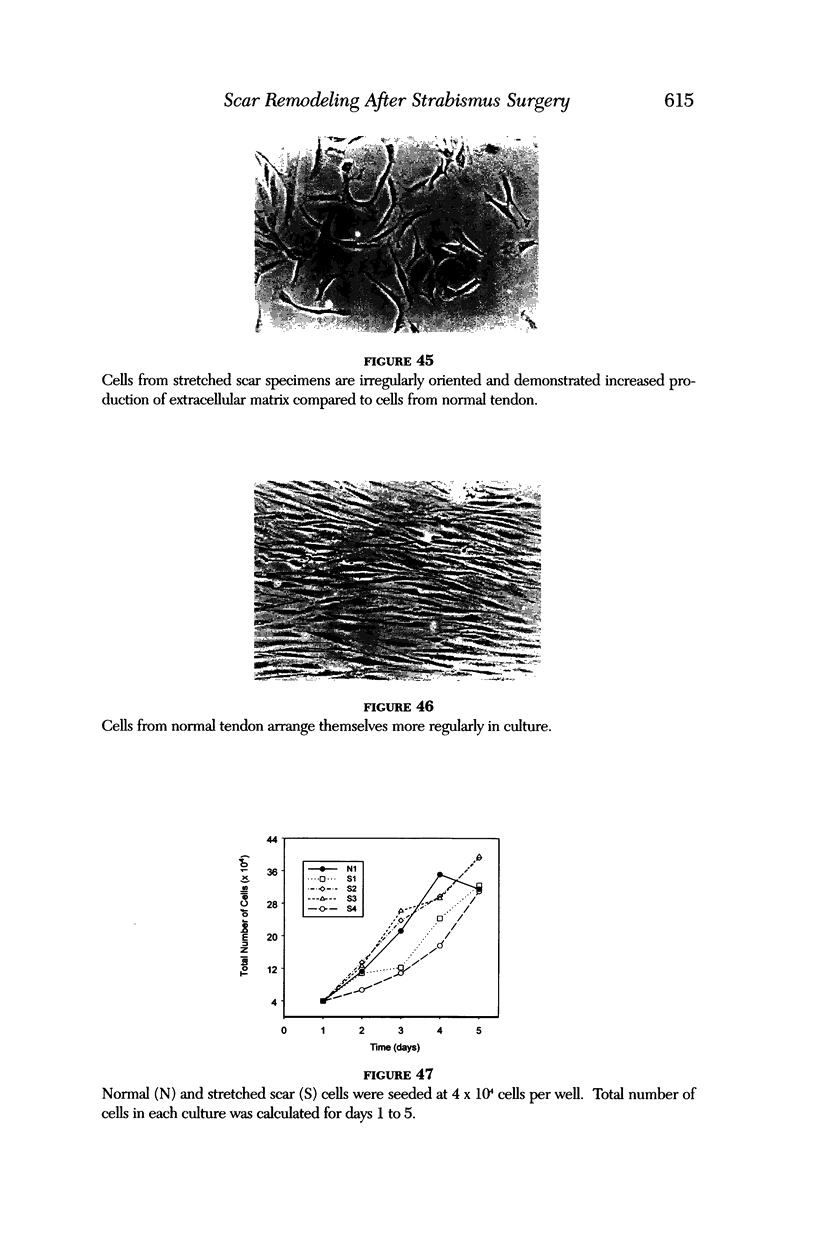
































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adloff M., Arnaud J. P. Surgical management of large incisional hernias by an intraperitoneal Mersilene mesh and an aponeurotic graft. Surg Gynecol Obstet. 1987 Sep;165(3):204–206. [PubMed] [Google Scholar]
- Aggarwal R. K., Willshaw H. E., Townsend P. New materials for rectus muscle tendon extension in strabismus surgery. Eye (Lond) 1993;7(Pt 1):40–42. doi: 10.1038/eye.1993.9. [DOI] [PubMed] [Google Scholar]
- Apt L., Gaffney W. L., Dora A. F. Experimental suture studies in strabismus surgery. I. Reattachment rate of extraocular muscles after recession and resection operations. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976 Nov 18;201(1):11–17. doi: 10.1007/BF00410143. [DOI] [PubMed] [Google Scholar]
- Bloom J. N., Parks M. M. The etiology, treatment and prevention of the "slipped muscle". J Pediatr Ophthalmol Strabismus. 1981 Jan-Feb;18(1):6–11. doi: 10.3928/0191-3913-19810101-03. [DOI] [PubMed] [Google Scholar]
- Bucknall T. E., Cox P. J., Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J (Clin Res Ed) 1982 Mar 27;284(6320):931–933. doi: 10.1136/bmj.284.6320.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucknall T. E., Ellis H. Abdominal wound closure--a comparison of monofilament nylon and polyglycolic acid. Surgery. 1981 Jun;89(6):672–677. [PubMed] [Google Scholar]
- Chantarasak N. D., Milner R. H. A comparison of scar quality in wounds closed under tension with PGA (Dexon) and Polydioxanone (PDS). Br J Plast Surg. 1989 Nov;42(6):687–691. doi: 10.1016/0007-1226(89)90082-9. [DOI] [PubMed] [Google Scholar]
- Cohen I. K., Keiser H. R. Disruption of healed scars in scurvy -- the result of a disequilibrium in collagen metabolism. Plast Reconstr Surg. 1976 Feb;57(2):213–215. doi: 10.1097/00006534-197602000-00015. [DOI] [PubMed] [Google Scholar]
- Cooney R., Iocono J., Maish G., Smith J. S., Ehrlich P. Tumor necrosis factor mediates impaired wound healing in chronic abdominal sepsis. J Trauma. 1997 Mar;42(3):415–420. doi: 10.1097/00005373-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Doillon C. J., Dunn M. G., Bender E., Silver F. H. Collagen fiber formation in repair tissue: development of strength and toughness. Coll Relat Res. 1985 Dec;5(6):481–492. doi: 10.1016/s0174-173x(85)80002-9. [DOI] [PubMed] [Google Scholar]
- Drews R. C. Polypropylene in the human eye. J Am Intraocul Implant Soc. 1983 Spring;9(2):137–142. doi: 10.1016/s0146-2776(83)80027-5. [DOI] [PubMed] [Google Scholar]
- Dunn M. G., Silver F. H. Viscoelastic behavior of human connective tissues: relative contribution of viscous and elastic components. Connect Tissue Res. 1983;12(1):59–70. doi: 10.3109/03008208309005612. [DOI] [PubMed] [Google Scholar]
- Elliot D., Mahaffey P. J. The stretched scar: the benefit of prolonged dermal support. Br J Plast Surg. 1989 Jan;42(1):74–78. doi: 10.1016/s0007-1226(89)90117-3. [DOI] [PubMed] [Google Scholar]
- Ellis H., Gajraj H., George C. D. Incisional hernias: when do they occur? Br J Surg. 1983 May;70(5):290–291. doi: 10.1002/bjs.1800700514. [DOI] [PubMed] [Google Scholar]
- Fischer J. D., Turner F. W. Abdominal incisional hernias: a ten-year review. Can J Surg. 1974 Jul;17(4):202–204. [PubMed] [Google Scholar]
- Gibson T., Kenedi R. M. Biomechanical properties of skin. Surg Clin North Am. 1967 Apr;47(2):279–294. doi: 10.1016/s0039-6109(16)38180-4. [DOI] [PubMed] [Google Scholar]
- Gomez D. E., Alonso D. F., Yoshiji H., Thorgeirsson U. P. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997 Oct;74(2):111–122. [PubMed] [Google Scholar]
- Gottrup F. Healing of incisional wounds in stomach and duodenum: influence of long-term healing on mechanical strength and collagen distribution. Acta Chir Scand. 1983;149(1):57–62. [PubMed] [Google Scholar]
- Harding K. G., Mudge M., Leinster S. J., Hughes L. E. Late development of incisional hernia: an unrecognised problem. Br Med J (Clin Res Ed) 1983 Feb 12;286(6364):519–520. doi: 10.1136/bmj.286.6364.519-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshowitz B., Lindenbaum E., Har-Shai Y. A skin-stretching device for the harnessing of the viscoelastic properties of skin. Plast Reconstr Surg. 1993 Aug;92(2):260–270. doi: 10.1097/00006534-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Jacob J. T., Lin J. J., Mikal S. P. Synthetic scleral reinforcement materials. III. Changes in surface and bulk physical properties. J Biomed Mater Res. 1997 Dec 15;37(4):525–533. doi: 10.1002/(sici)1097-4636(19971215)37:4<525::aid-jbm11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Jonsson T., Högström H. Neutrophil-dependent decrease in early wound margin strength. Arch Surg. 1991 Nov;126(11):1423–1426. doi: 10.1001/archsurg.1991.01410350117019. [DOI] [PubMed] [Google Scholar]
- Kenedi R. M., Gibson T., Daly C. H., Abrahams M. Biomechanical characteristics of human skin and costal cartilage. Fed Proc. 1966 May-Jun;25(3):1084–1087. [PubMed] [Google Scholar]
- Ketchum L. D., Martin N. L., Kappel D. A. Experimental evaluation of factors affecting the strength of tendon repairs. Plast Reconstr Surg. 1977 May;59(5):708–719. doi: 10.1097/00006534-197705000-00014. [DOI] [PubMed] [Google Scholar]
- Kleiner D. E., Stetler-Stevenson W. G. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994 May 1;218(2):325–329. doi: 10.1006/abio.1994.1186. [DOI] [PubMed] [Google Scholar]
- LEVENSON S. M., GEEVER E. F., CROWLEY L. V., OATES J. F., 3rd, BERARD C. W., ROSEN H. THE HEALING OF RAT SKIN WOUNDS. Ann Surg. 1965 Feb;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. Y., Glagov S., Mathews M. B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976 Feb 6;191(4226):475–477. doi: 10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- Liang M. D., Briggs P., Heckler F. R., Futrell J. W. Presuturing--a new technique for closing large skin defects: clinical and experimental studies. Plast Reconstr Surg. 1988 May;81(5):694–702. doi: 10.1097/00006534-198805000-00008. [DOI] [PubMed] [Google Scholar]
- McGaw W. T., Ten Cate A. R. A role for collagen phagocytosis by fibroblasts in scar remodeling: an ultrastructural stereologic study. J Invest Dermatol. 1983 Oct;81(4):375–378. doi: 10.1111/1523-1747.ep12519983. [DOI] [PubMed] [Google Scholar]
- Meikle M. C., Sellers A., Reynolds J. J. Effect of tensile mechanical stress on the synthesis of metalloproteinases by rabbit coronal sutures in vitro. Calcif Tissue Int. 1980;30(1):77–82. doi: 10.1007/BF02408610. [DOI] [PubMed] [Google Scholar]
- Meyer M., McGrouther D. A. A study relating wound tension to scar morphology in the pre-sternal scar using Langers technique. Br J Plast Surg. 1991 May-Jun;44(4):291–294. doi: 10.1016/0007-1226(91)90074-t. [DOI] [PubMed] [Google Scholar]
- Mooney D. P., O'Reilly M., Gamelli R. L. Tumor necrosis factor and wound healing. Ann Surg. 1990 Feb;211(2):124–129. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge M., Hughes L. E. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985 Jan;72(1):70–71. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Nordström R. E., Nordström R. M. Absorbable versus nonabsorbable sutures to prevent postoperative stretching of wound area. Plast Reconstr Surg. 1986 Aug;78(2):186–190. [PubMed] [Google Scholar]
- Parks M. M., Bloom J. N. The "slipped" muscle. Ophthalmology. 1979 Aug;86(8):1389–1396. doi: 10.1016/s0161-6420(79)35386-6. [DOI] [PubMed] [Google Scholar]
- Pitelka D. R., Taggart B. N. Mechanical tension induces lateral movement of intramembrane components of the tight junction: studies on mouse mammary cells in culture. J Cell Biol. 1983 Mar;96(3):606–612. doi: 10.1083/jcb.96.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantner J. J., Smine A., Quinn T. A. Matrix metalloproteinases and metalloproteinase inhibitors in human interphotoreceptor matrix and vitreous. Curr Eye Res. 1998 Feb;17(2):132–140. doi: 10.1076/ceyr.17.2.132.5610. [DOI] [PubMed] [Google Scholar]
- Playforth M. J., Sauven P. D., Evans M., Pollock A. V. The prediction of incisional hernias by radio-opaque markers. Ann R Coll Surg Engl. 1986 Mar;68(2):82–84. [PMC free article] [PubMed] [Google Scholar]
- Pollock A. V. Laparotomy. J R Soc Med. 1981 Jul;74(7):480–484. doi: 10.1177/014107688107400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez O. M. The anchor subperiosteal forehead lift. Plast Reconstr Surg. 1995 May;95(6):993–1006. [PubMed] [Google Scholar]
- Rapala K., Laato M., Niinikoski J., Kujari H., Söder O., Mauviel A., Pujol J. P. Tumor necrosis factor alpha inhibits wound healing in the rat. Eur Surg Res. 1991;23(5-6):261–268. doi: 10.1159/000129163. [DOI] [PubMed] [Google Scholar]
- Regan M. C., Kirk S. J., Hurson M., Sodeyama M., Wasserkrug H. L., Barbul A. Tumor necrosis factor-alpha inhibits in vivo collagen synthesis. Surgery. 1993 Feb;113(2):173–177. [PubMed] [Google Scholar]
- Repka M. X., Fishman P. J., Guyton D. L. The site of reattachment of the extraocular muscle following hang-back recession. J Pediatr Ophthalmol Strabismus. 1990 Nov-Dec;27(6):286–290. doi: 10.3928/0191-3913-19901101-04. [DOI] [PubMed] [Google Scholar]
- Santora T. A., Roslyn J. J. Incisional hernia. Surg Clin North Am. 1993 Jun;73(3):557–570. doi: 10.1016/s0039-6109(16)46037-8. [DOI] [PubMed] [Google Scholar]
- Sanz L. E., Patterson J. A., Kamath R., Willett G., Ahmed S. W., Butterfield A. B. Comparison of Maxon suture with Vicryl, chromic catgut, and PDS sutures in fascial closure in rats. Obstet Gynecol. 1988 Mar;71(3 Pt 1):418–422. [PubMed] [Google Scholar]
- Sauter E., Thibodeaux K., Myers B. Effect of high tension and relaxing incisions on wound healing in rats. South Med J. 1985 Dec;78(12):1451–1453. doi: 10.1097/00007611-198512000-00013. [DOI] [PubMed] [Google Scholar]
- Schoetz D. J., Jr, Coller J. A., Veidenheimer M. C. Closure of abdominal wounds with polydioxanone. A prospective study. Arch Surg. 1988 Jan;123(1):72–74. doi: 10.1001/archsurg.1988.01400250082015. [DOI] [PubMed] [Google Scholar]
- Scott A. B., Collins C. C., O'Meara D. M. A forceps to measure strabismus forces. Arch Ophthalmol. 1972 Sep;88(3):330–333. doi: 10.1001/archopht.1972.01000030332022. [DOI] [PubMed] [Google Scholar]
- Shauly Y., Prager T. C., Mazow M. L. Clinical characteristics and long-term postoperative results of infantile esotropia. Am J Ophthalmol. 1994 Feb 15;117(2):183–189. doi: 10.1016/s0002-9394(14)73075-2. [DOI] [PubMed] [Google Scholar]
- Simpson D. M., Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972 Aug;51(8):2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerlad B. C., Creasey J. M. The stretched scar: a clinical and histological study. Br J Plast Surg. 1978 Jan;31(1):34–45. doi: 10.1016/0007-1226(78)90012-7. [DOI] [PubMed] [Google Scholar]
- Sprunger D. T., Helveston E. M. Progressive overcorrection after inferior rectus recession. J Pediatr Ophthalmol Strabismus. 1993 May-Jun;30(3):145–148. doi: 10.3928/0191-3913-19930501-04. [DOI] [PubMed] [Google Scholar]
- Squier C. A. The stretching of mouse skin in vivo: effect on epidermal proliferation and thickness. J Invest Dermatol. 1980 Feb;74(2):68–71. doi: 10.1111/1523-1747.ep12519868. [DOI] [PubMed] [Google Scholar]
- TOUR R. L., ASBURY T. Overcorrection of esotropia following bilateral five-mm, medial rectus recession. Am J Ophthalmol. 1958 May;45(5):644–653. [PubMed] [Google Scholar]
- Thomson S. R., Gregory M. A., Mars M., Natasen J., Naicker T., Baker L. W. Morphological aspects of microarterial anastomoses: a comparison of nylon with polydioxanone. Br J Plast Surg. 1995 Apr;48(3):165–171. doi: 10.1016/0007-1226(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Tyrell J., Silberman H., Chandrasoma P., Niland J., Shull J. Absorbable versus permanent mesh in abdominal operations. Surg Gynecol Obstet. 1989 Mar;168(3):227–232. [PubMed] [Google Scholar]
- Urschel J. D., Scott P. G., Williams H. T. Etiology of late developing incisional hernias--the possible role of mechanical stress. Med Hypotheses. 1988 Jan;25(1):31–34. doi: 10.1016/0306-9877(88)90043-6. [DOI] [PubMed] [Google Scholar]
- Von Noorden G. K., Isaza A., Parks M. E. Surgical treatment of congenital esotropia. Trans Am Acad Ophthalmol Otolaryngol. 1972 Nov-Dec;76(6):1465–1478. [PubMed] [Google Scholar]
- Wantz G. E. Incisional hernioplasty with Mersilene. Surg Gynecol Obstet. 1991 Feb;172(2):129–137. [PubMed] [Google Scholar]
- Weakley D. R., Jr, Parks M. M. Results from 7-mm bilateral recessions of the medial rectus muscles for congenital esotropia. Ophthalmic Surg. 1990 Dec;21(12):827–830. [PubMed] [Google Scholar]
- Windsor J. A., Knight G. S., Hill G. L. Wound healing response in surgical patients: recent food intake is more important than nutritional status. Br J Surg. 1988 Feb;75(2):135–137. doi: 10.1002/bjs.1800750215. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970 Apr;26(4):507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Gelberman R. H., Cobb N. G., Amiel D., Lothringer K., Akeson W. H. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981 Dec;52(6):615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]