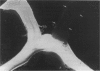
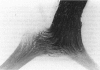
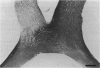
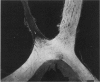
Abstract
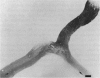
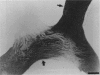
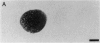
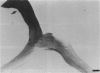
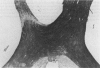
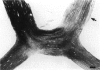
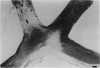
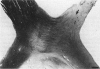
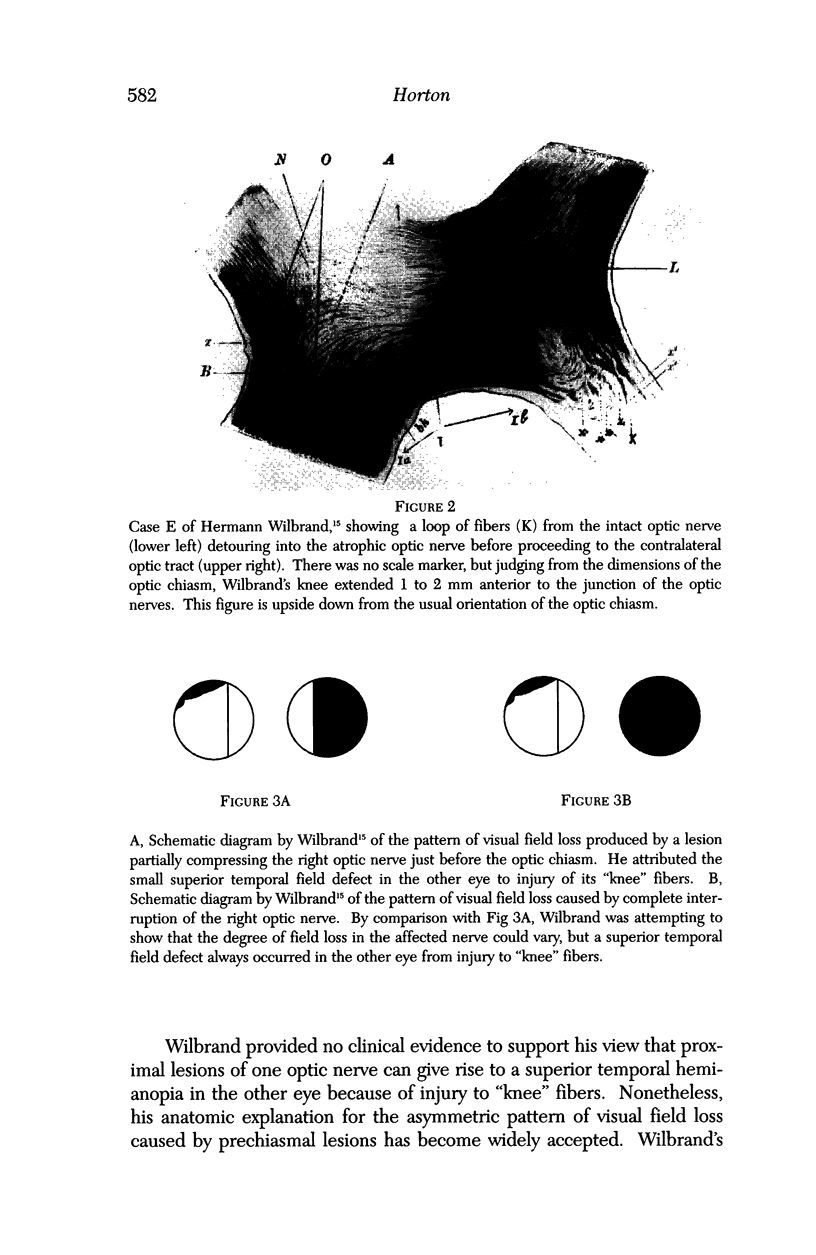
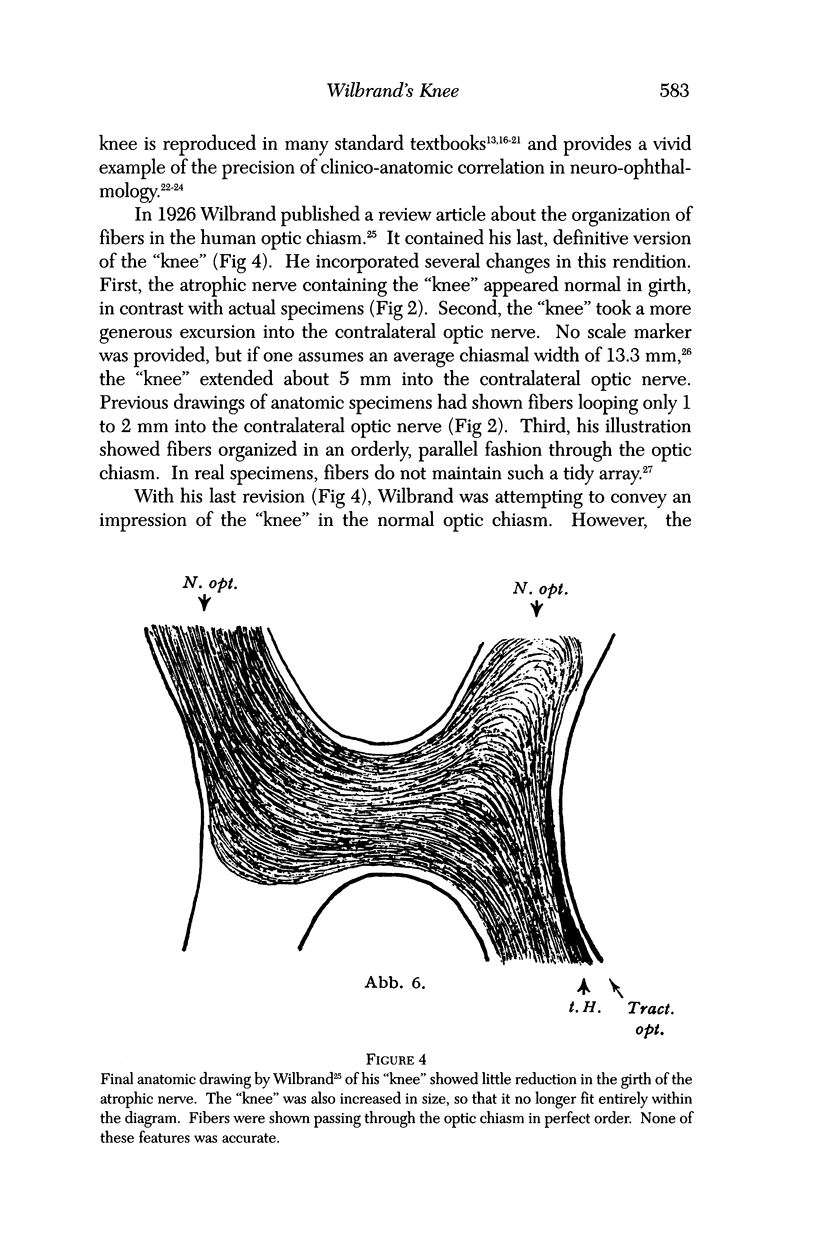
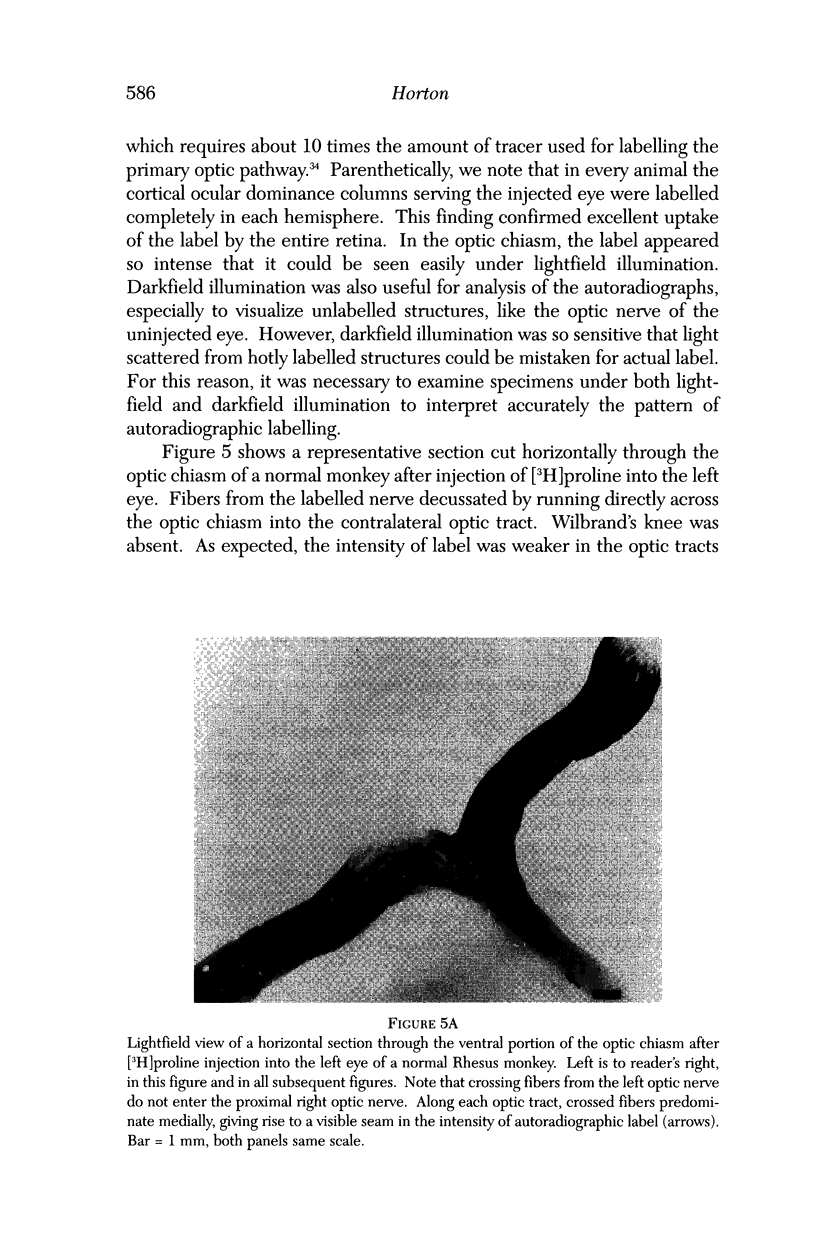
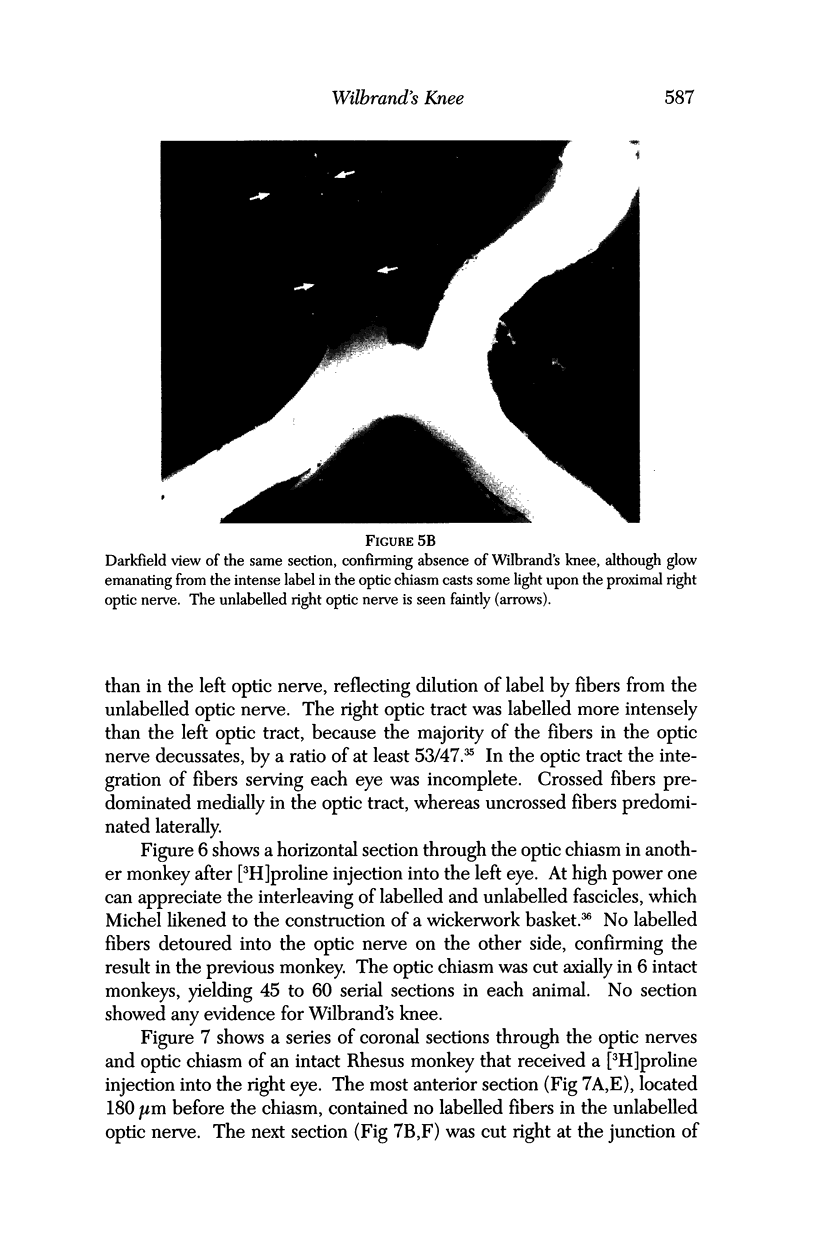
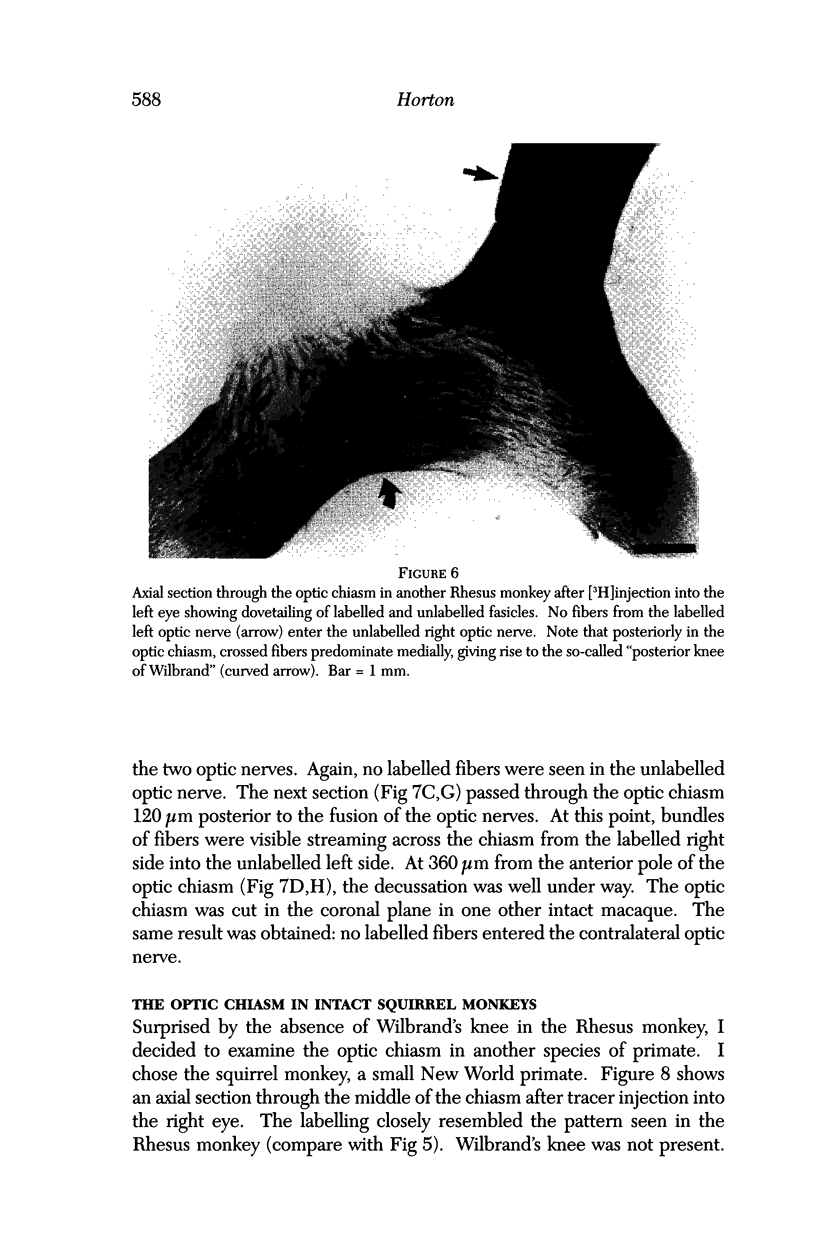
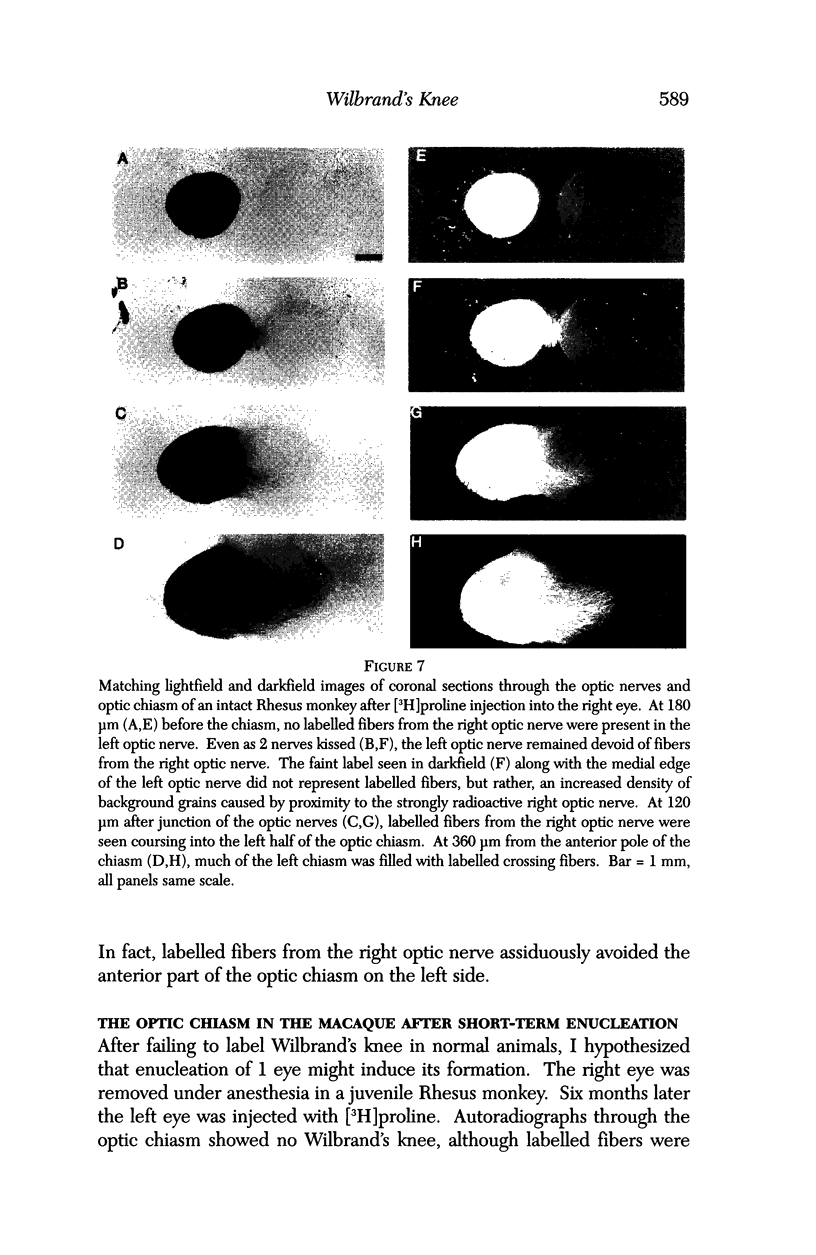
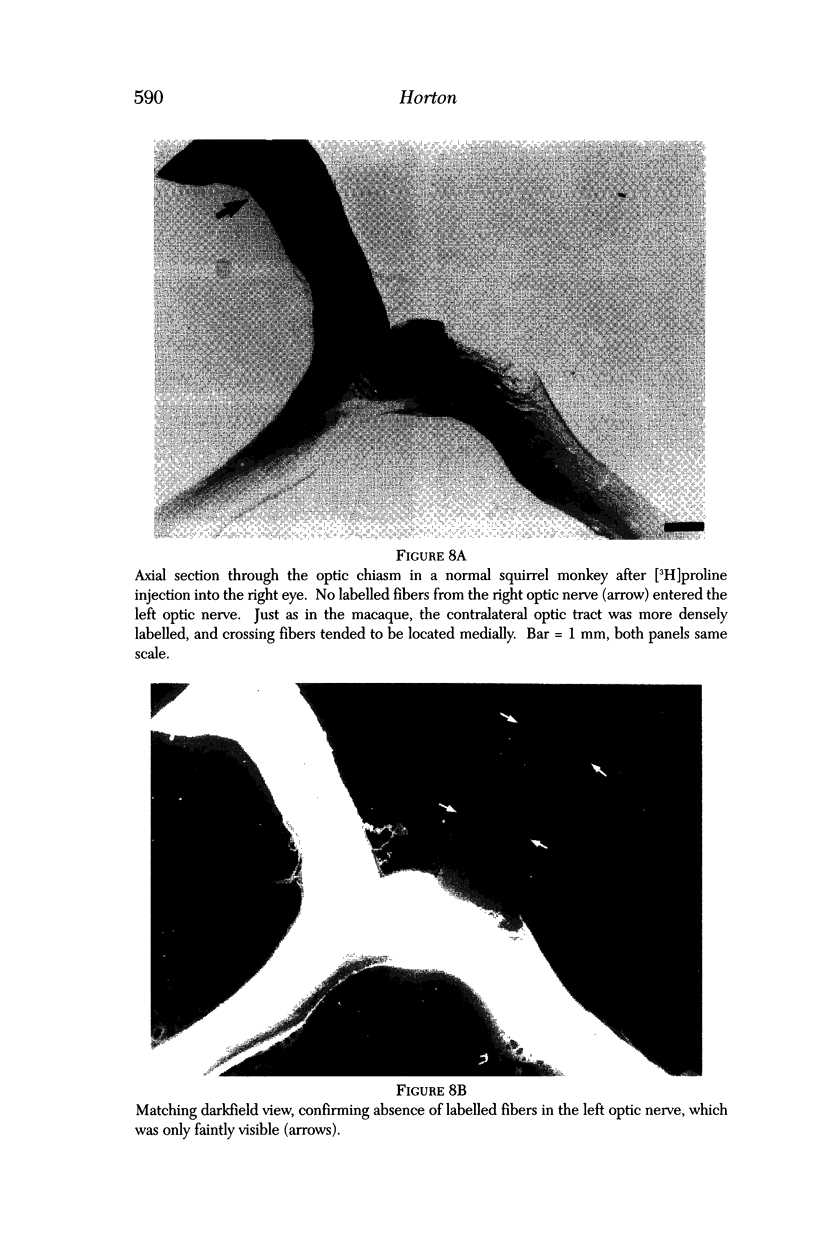
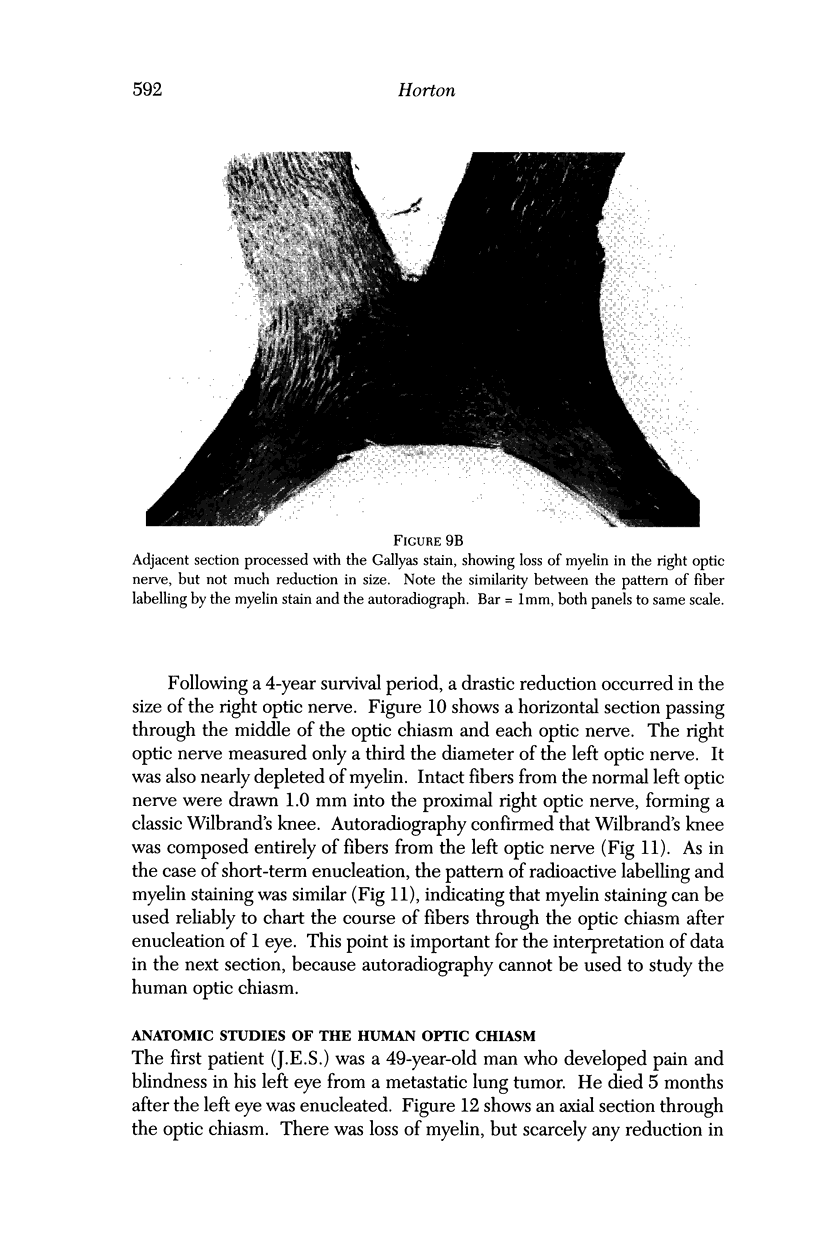
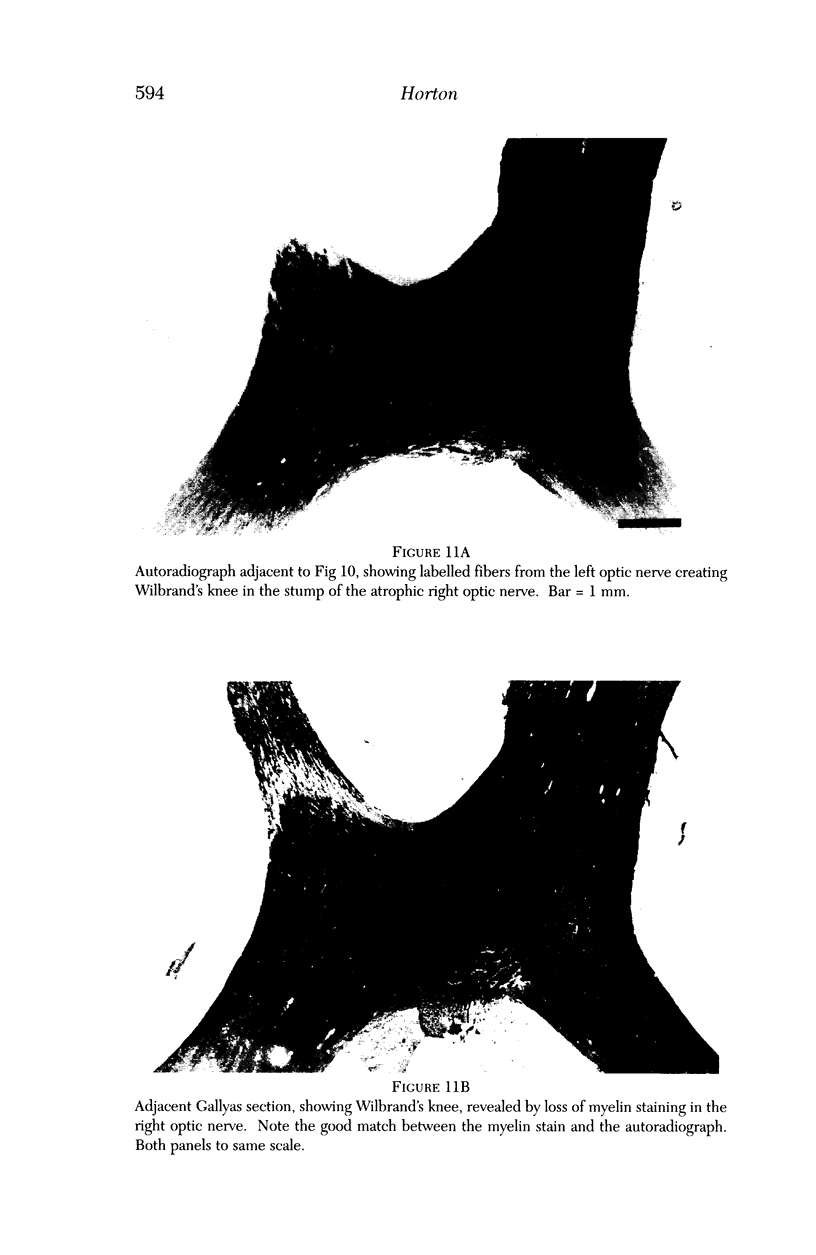
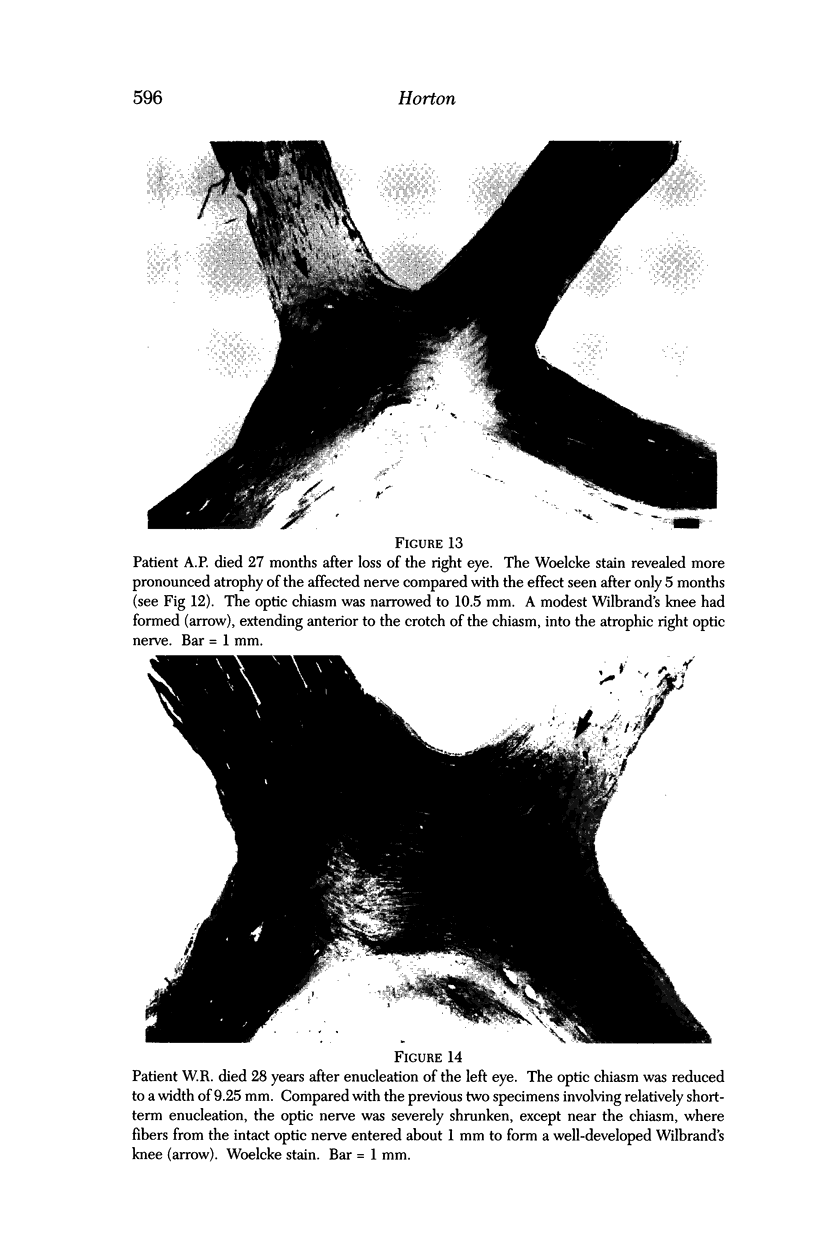
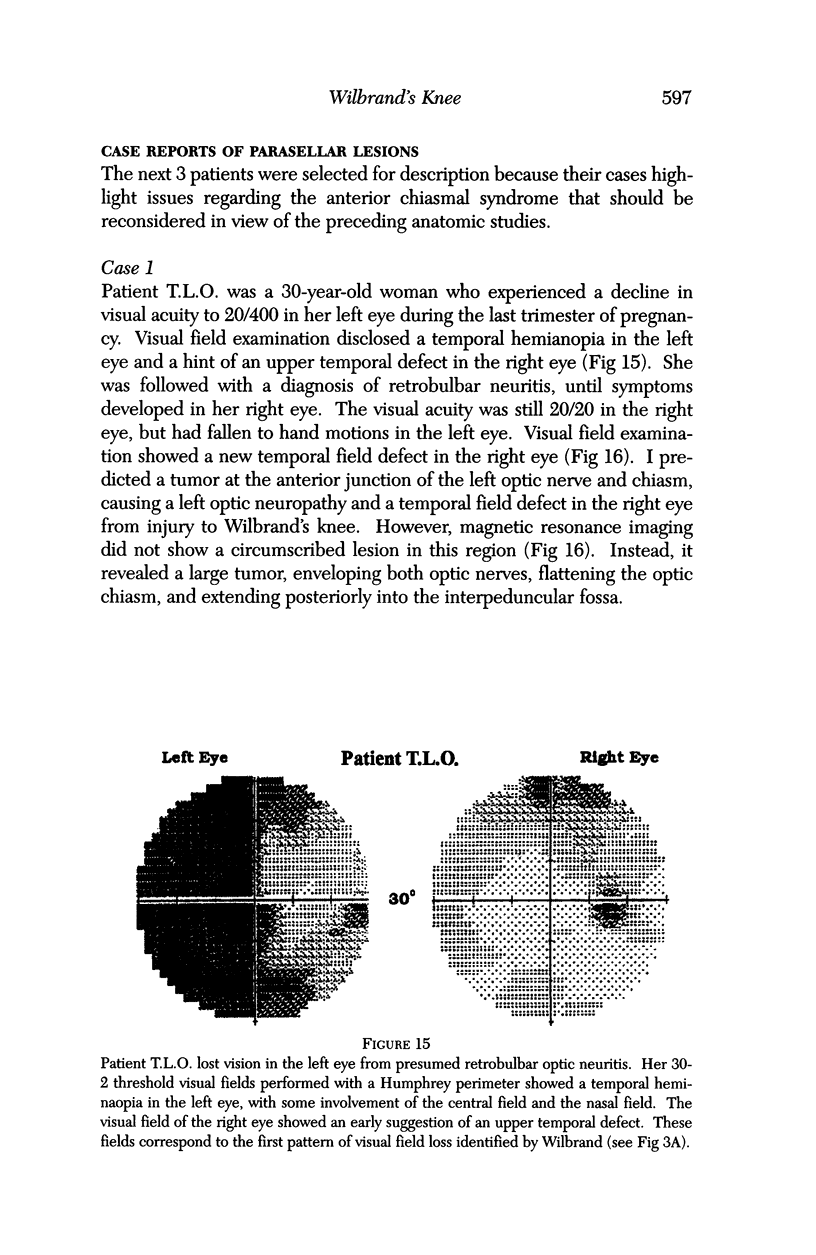
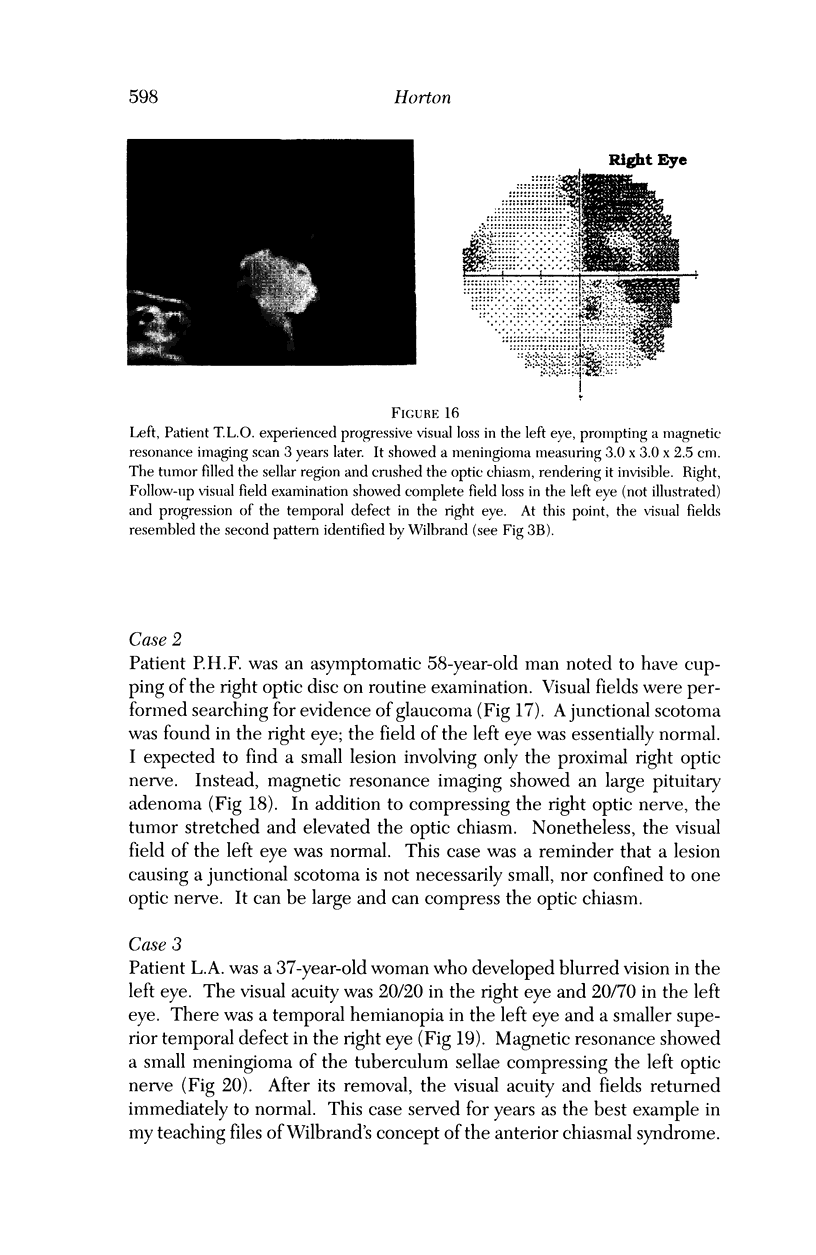
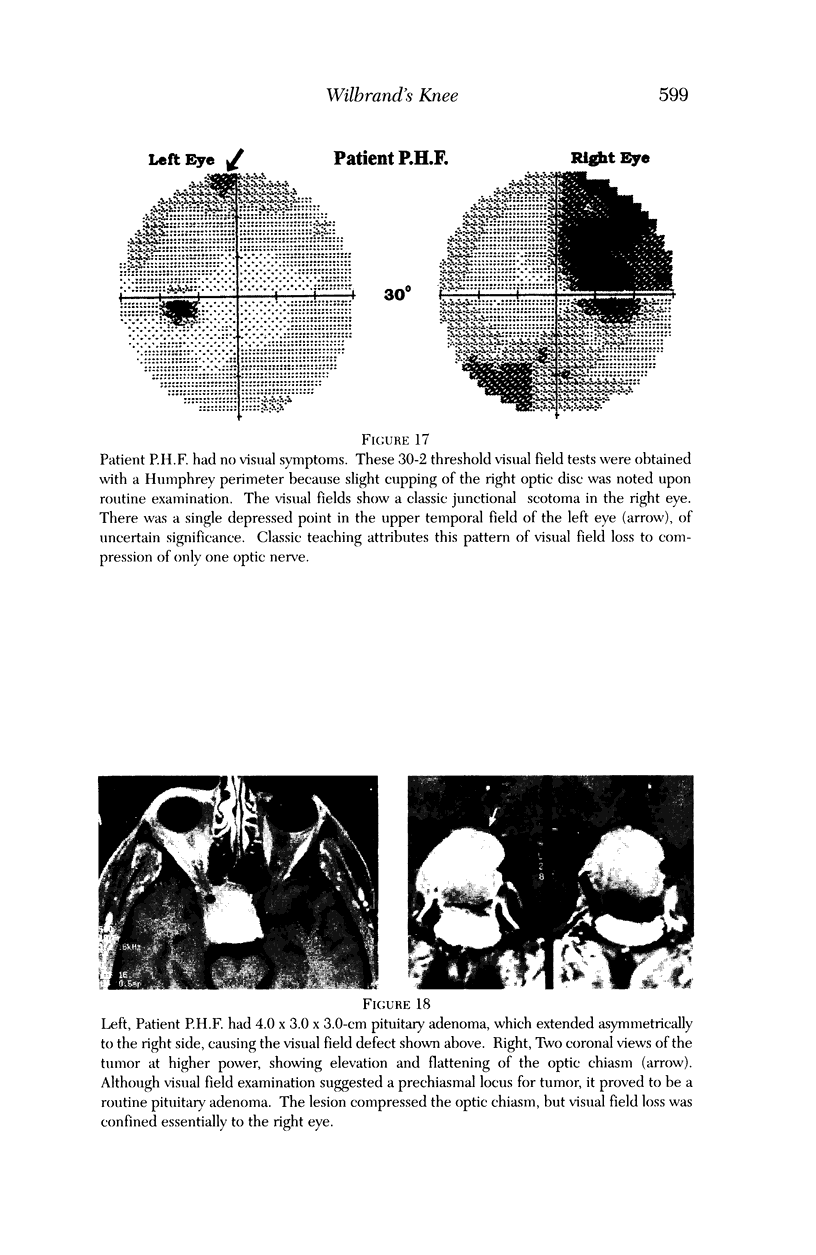
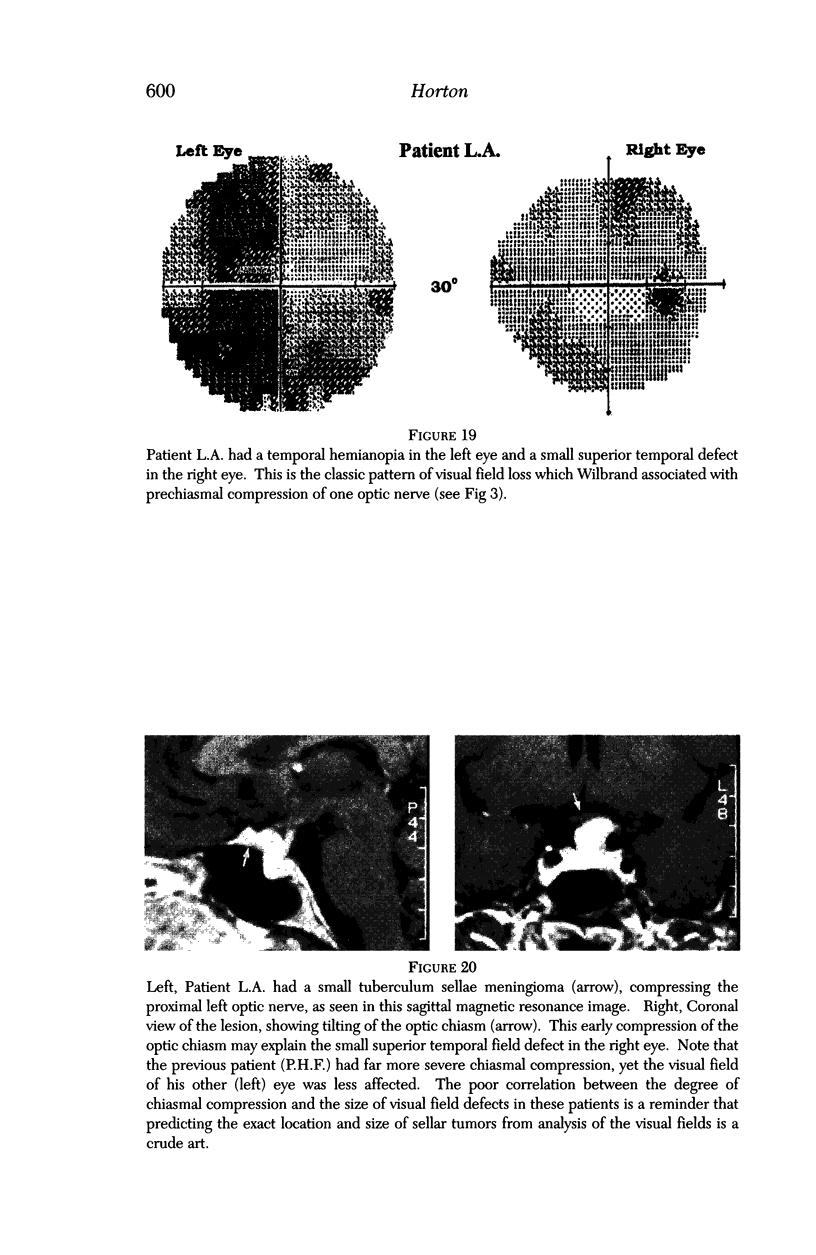
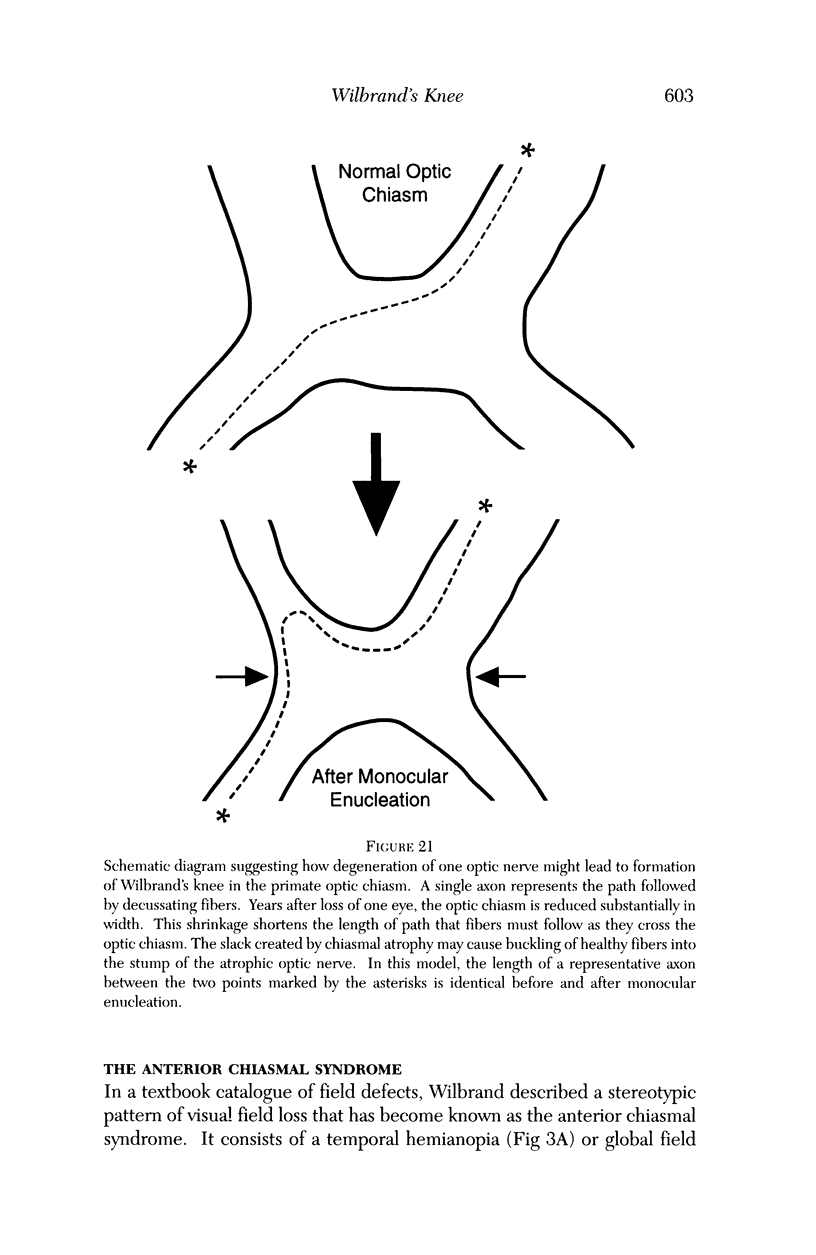
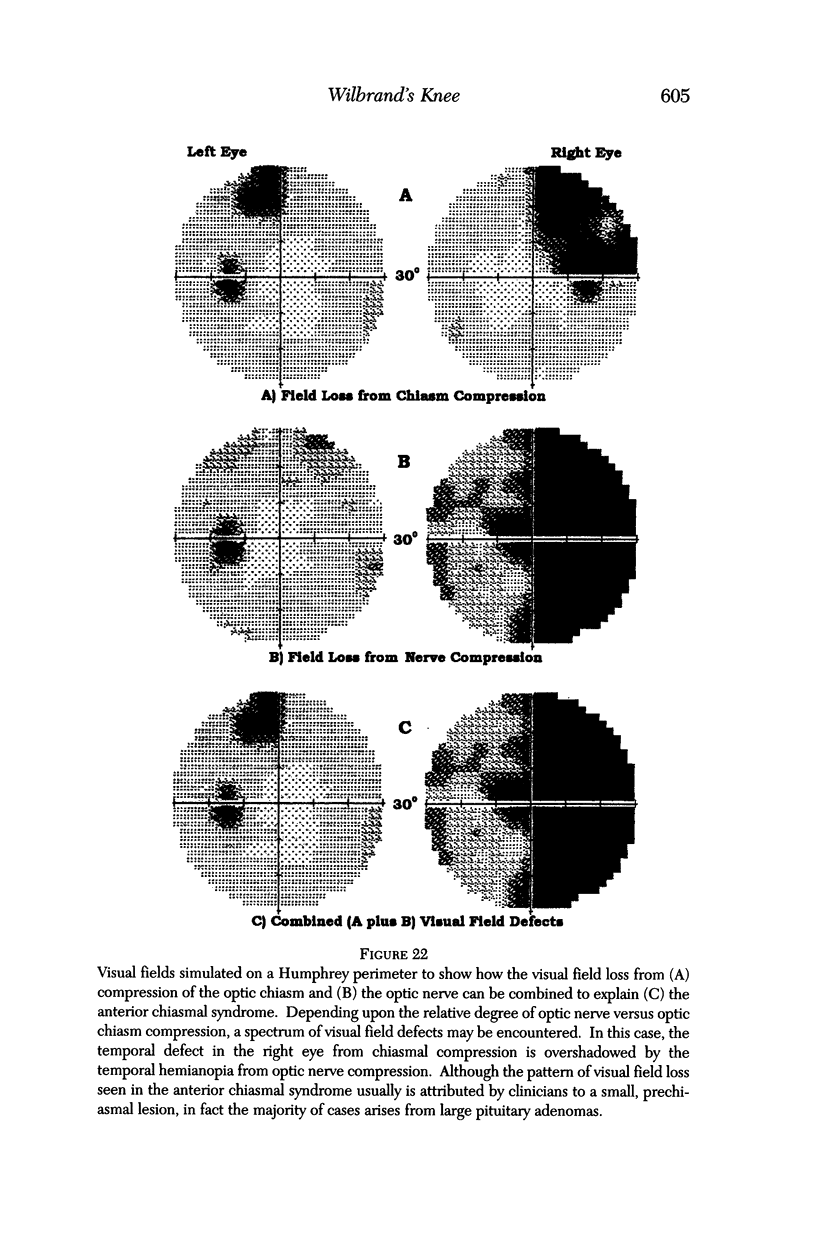
PURPOSE: The anterior chiasmal syndrome consists of a temporal hemianopia or complete visual field loss in one eye, plus a superior temporal hemianopia in the other eye. The superior temporal hemianopia in the other eye is thought to result from injury to Wilbrand's Knee of the optic chiasm. Wilbrand's Knee is a loop of decussating fibers which detours into the contralateral optic nerve before entering the optic tract. I studied the organization of fibers in the optic chiasm of monkeys and humans to verify the existence of Wilbrand's Knee and to elucidate further the pattern of visual field loss seen from lesions of the sellar region. METHODS: The primary optic pathway was labelled in monkeys by injection of [3H] proline into one eye, followed by autoradiography. There were 8 intact Rhesus monkeys and 3 intact squirrel monkeys. In addition, the optic pathway was studied in the Rhesus monkey 6 months and 4 years after monocular enucleation. The optic chiasm was also examined using myelin stains in specimens obtained post-mortem from 3 patients. The patients had lost 1 eye 5 months, 2 years, and 28 years prior to their deaths. Finally, clinical observations were recorded in 3 patients with the anterior chiasmal syndrome. RESULTS: In normal Rhesus and squirrel monkeys, optic nerve fibers crossed the optic chiasm without entering the contralateral optic nerve. After short-term monocular enucleation, fibers from the normal optic nerve were drawn closer to the entry zone of the degenerating optic nerve, but Wilbrand's Knee was still absent. After long-term enucleation, a typical Wilbrand's Knee was induced to form. In the human, Wilbrand's Knee was absent 5 months after monocular enucleation, but emerged in the two cases involving long-term enucleation, in a fashion analogous to the monkey. The case reports describe 3 patients with variants of the anterior chiasmal syndrome from parasellar tumors. CONCLUSIONS: Wilbrand's Knee does not exist in the normal primate optic chiasm. It forms gradually over a period of years following monocular enucleation, presumably from shrinkage of the optic chiasm caused by atrophy of fibers from the enucleated eye. Therefore, the superior temporal hemianopia in the "other eye" seen in the anterior chiasmal syndrome cannot be due to compression of Wilbrand's Knee. I propose that it occurs from combined compression of the optic chiasm and one (or both) optic nerves.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. C. Field loss due to lesions at the anterior angle of the chiasm. Proc R Soc Med. 1972 Jun;65(6):519–520. [PMC free article] [PubMed] [Google Scholar]
- Colello R. J., Guillery R. W. The early development of retinal ganglion cells with uncrossed axons in the mouse: retinal position and axonal course. Development. 1990 Mar;108(3):515–523. doi: 10.1242/dev.108.3.515. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1(2):203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Godement P., Vanselow J., Thanos S., Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987 Dec;101(4):697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- HOYT W. F., LUIS O. The primate chiasm. Details of visual fiber organization studied by silver impregnation techniques. Arch Ophthalmol. 1963 Jul;70:69–85. doi: 10.1001/archopht.1963.00960050071013. [DOI] [PubMed] [Google Scholar]
- Hedges T. R. Preservation of the upper nasal field in the chiasmal syndrome: an anatomic explanation. Trans Am Ophthalmol Soc. 1969;67:131–141. [PMC free article] [PubMed] [Google Scholar]
- Hershenfeld S. A., Sharpe J. A. Monocular temporal hemianopia. Br J Ophthalmol. 1993 Jul;77(7):424–427. doi: 10.1136/bjo.77.7.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Greenwood M. M., Hubel D. H. Non-retinotopic arrangement of fibres in cat optic nerve. Nature. 1979 Dec 13;282(5740):720–722. doi: 10.1038/282720a0. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hocking D. R. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. J Neurosci. 1996 Mar 1;16(5):1791–1807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Hocking D. R. Anatomical demonstration of ocular dominance columns in striate cortex of the squirrel monkey. J Neurosci. 1996 Sep 1;16(17):5510–5522. doi: 10.1523/JNEUROSCI.16-17-05510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Hocking D. R. Intrinsic variability of ocular dominance column periodicity in normal macaque monkeys. J Neurosci. 1996 Nov 15;16(22):7228–7239. doi: 10.1523/JNEUROSCI.16-22-07228.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Hocking D. R. Timing of the critical period for plasticity of ocular dominance columns in macaque striate cortex. J Neurosci. 1997 May 15;17(10):3684–3709. doi: 10.1523/JNEUROSCI.17-10-03684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer C., Chumbley L., Downer J. C. Quantitative histology of optic nerve, optic tract and lateral geniculate nucleus of man. J Anat. 1967 Jun;101(Pt 3):393–401. [PMC free article] [PubMed] [Google Scholar]
- Meissirel C., Chalupa L. M. Organization of pioneer retinal axons within the optic tract of the rhesus monkey. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3906–3910. doi: 10.1073/pnas.91.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito J. Retinogeniculate projection fibers in the monkey optic chiasm: a demonstration of the fiber arrangement by means of wheat germ agglutinin conjugated to horseradish peroxidase. J Comp Neurol. 1994 Aug 22;346(4):559–571. doi: 10.1002/cne.903460408. [DOI] [PubMed] [Google Scholar]
- Newman S. A., Miller N. R. Optic tract syndrome. Neuro-ophthalmologic considerations. Arch Ophthalmol. 1983 Aug;101(8):1241–1250. doi: 10.1001/archopht.1983.01040020243018. [DOI] [PubMed] [Google Scholar]
- Reese B. E., Baker G. E. Changes in fiber organization within the chiasmatic region of mammals. Vis Neurosci. 1992 Dec;9(6):527–533. doi: 10.1017/s0952523800001772. [DOI] [PubMed] [Google Scholar]
- Reese B. E. Clinical implications of the fibre order in the optic pathway of primates. Neurol Res. 1993 Apr;15(2):83–86. doi: 10.1080/01616412.1993.11740114. [DOI] [PubMed] [Google Scholar]
- Reese B. E., Cowey A. Fibre organization of the monkey's optic tract: II. Noncongruent representation of the two half-retinae. J Comp Neurol. 1990 May 15;295(3):401–412. doi: 10.1002/cne.902950305. [DOI] [PubMed] [Google Scholar]
- Richter C. P., Warner C. L. Comparison of Weigert stained sections with unfixed, unstained sections for study of myelin sheaths. Proc Natl Acad Sci U S A. 1974 Mar;71(3):598–601. doi: 10.1073/pnas.71.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino P. J., Paris M., Schatz N. J., Orr L. S., Corbett J. J. Optic tract syndrome. A review of 21 patients. Arch Ophthalmol. 1978 Apr;96(4):656–663. doi: 10.1001/archopht.1978.03910050352011. [DOI] [PubMed] [Google Scholar]
- Sretavan D. W. Pathfinding at the mammalian optic chiasm. Curr Opin Neurobiol. 1993 Feb;3(1):45–52. doi: 10.1016/0959-4388(93)90034-v. [DOI] [PubMed] [Google Scholar]
- Sretavan D. W., Reichardt L. F. Time-lapse video analysis of retinal ganglion cell axon pathfinding at the mammalian optic chiasm: growth cone guidance using intrinsic chiasm cues. Neuron. 1993 Apr;10(4):761–777. doi: 10.1016/0896-6273(93)90176-r. [DOI] [PubMed] [Google Scholar]
- Trobe J. D., Tao A. H., Schuster J. J. Perichiasmal tumors: diagnostic and prognostic features. Neurosurgery. 1984 Sep;15(3):391–399. doi: 10.1227/00006123-198409000-00016. [DOI] [PubMed] [Google Scholar]
- Vinger P. F., Seelenfreund M. H. Damage to anterior-loop fibers of optic chiasm. Am J Ophthalmol. 1969 Oct;68(4):630–633. doi: 10.1016/0002-9394(69)91242-2. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]