Abstract
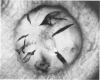
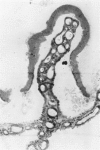
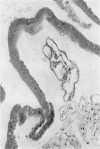
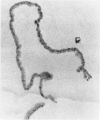
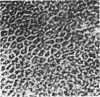
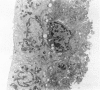
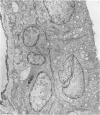
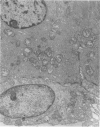
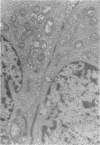
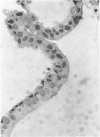
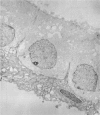
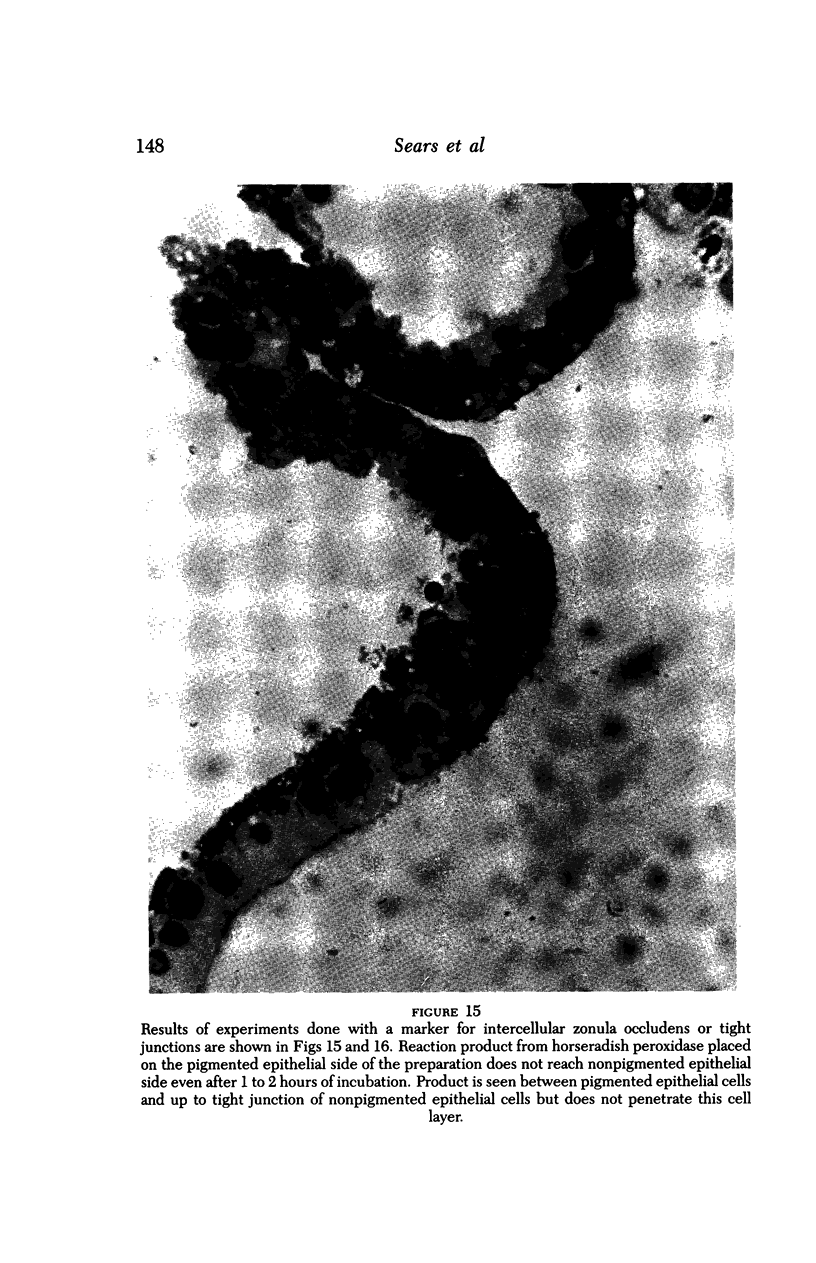
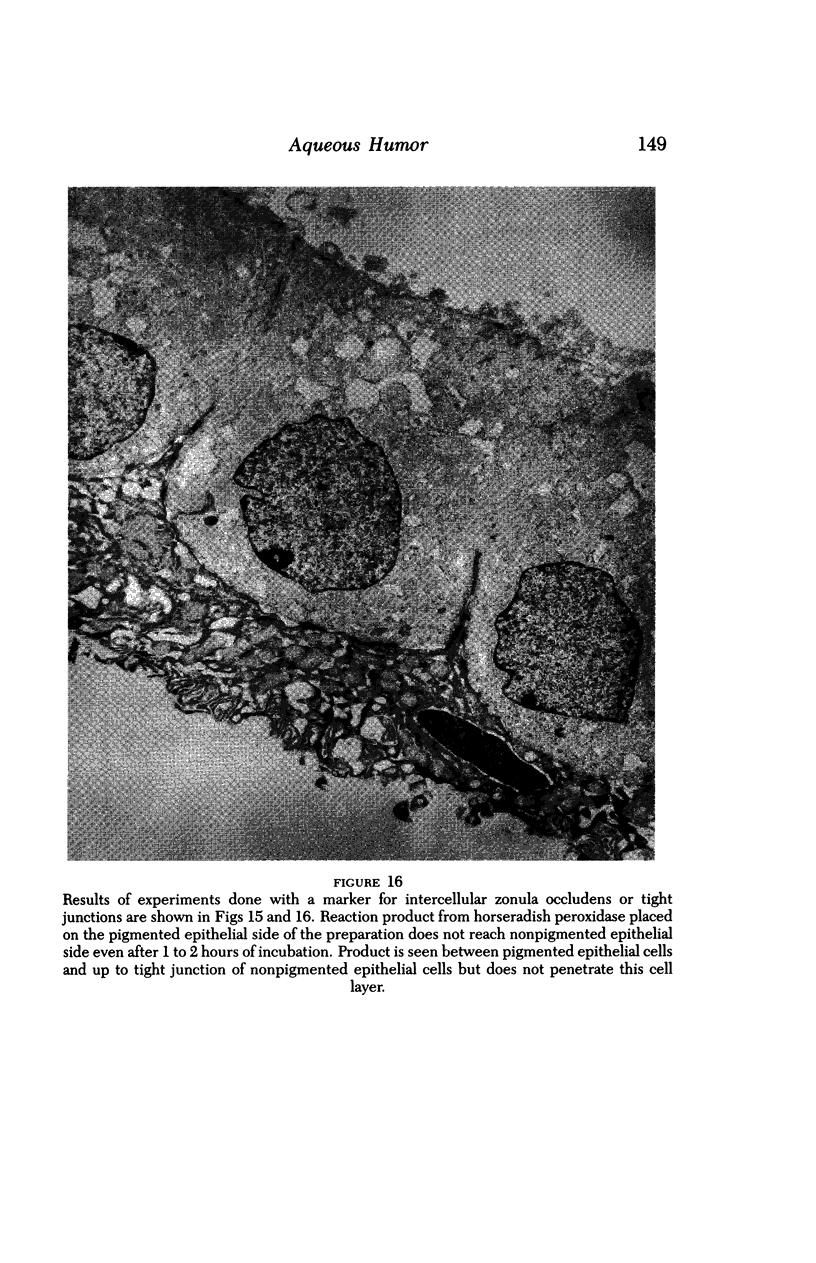
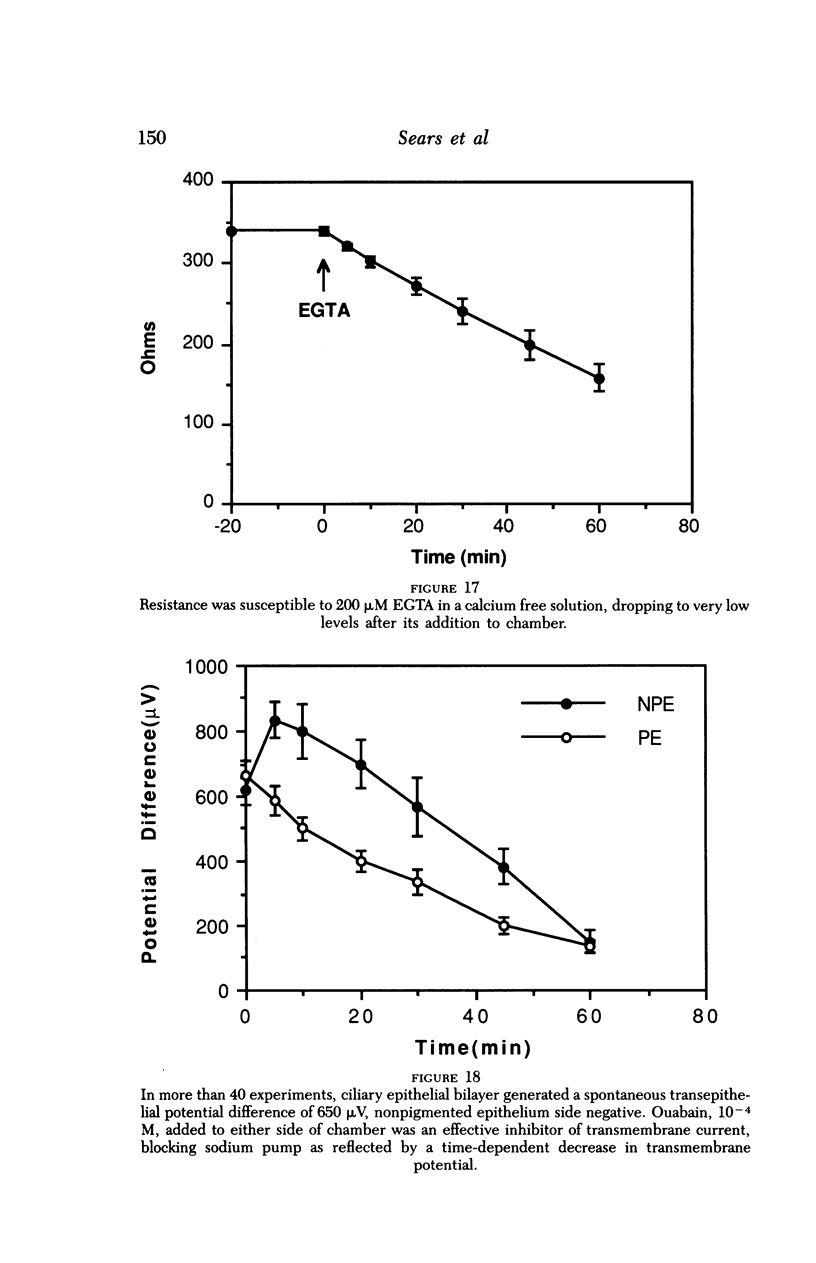
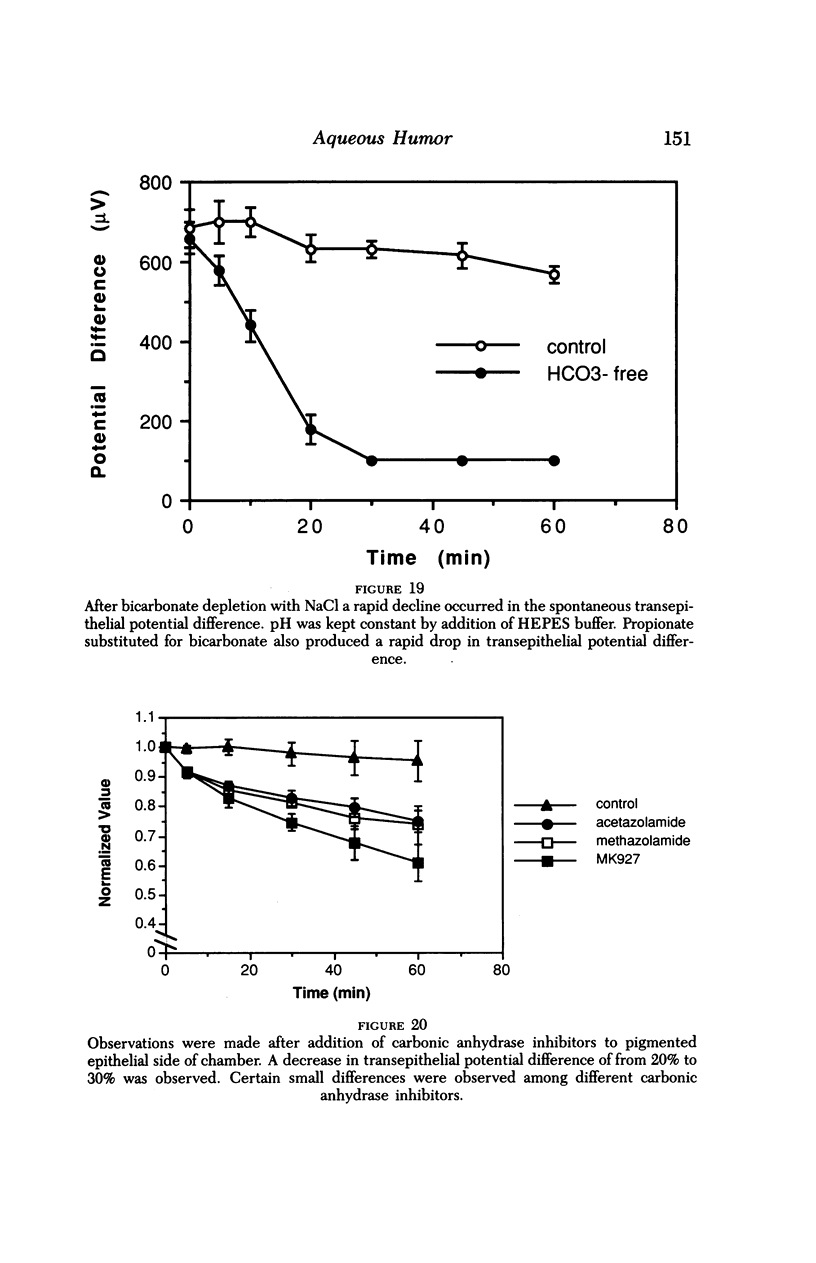
An intact ciliary epithelial bilayer has been isolated from the rabbit eye by perfusion, microsurgical dissection, and recovery techniques. Vital subcellular organelles and intercellular junctions of this epithelial bilayer preparation are very well preserved. The total electrical resistance of the epithelial bilayer is 350 ohms, and the transepithelial potential is 650 microV, nonpigmented epithelium side negative. The electrical resistance is reduced by 0.2 mM EGTA and the transepithelial potential reduced by 0.1 mM ouabain. Bicarbonate depletion at a constant pH of 7.4 rapidly and significantly reduces the transepithelial potential. Carbonic anhydrase inhibitors decrease transmembrane potential by as much as 30%. These morphologic and physiologic experiments authenticate the validity of this bilayered epithelial preparation for future use in detailed studies of the mechanism of aqueous humor formation.
Full text
PDF




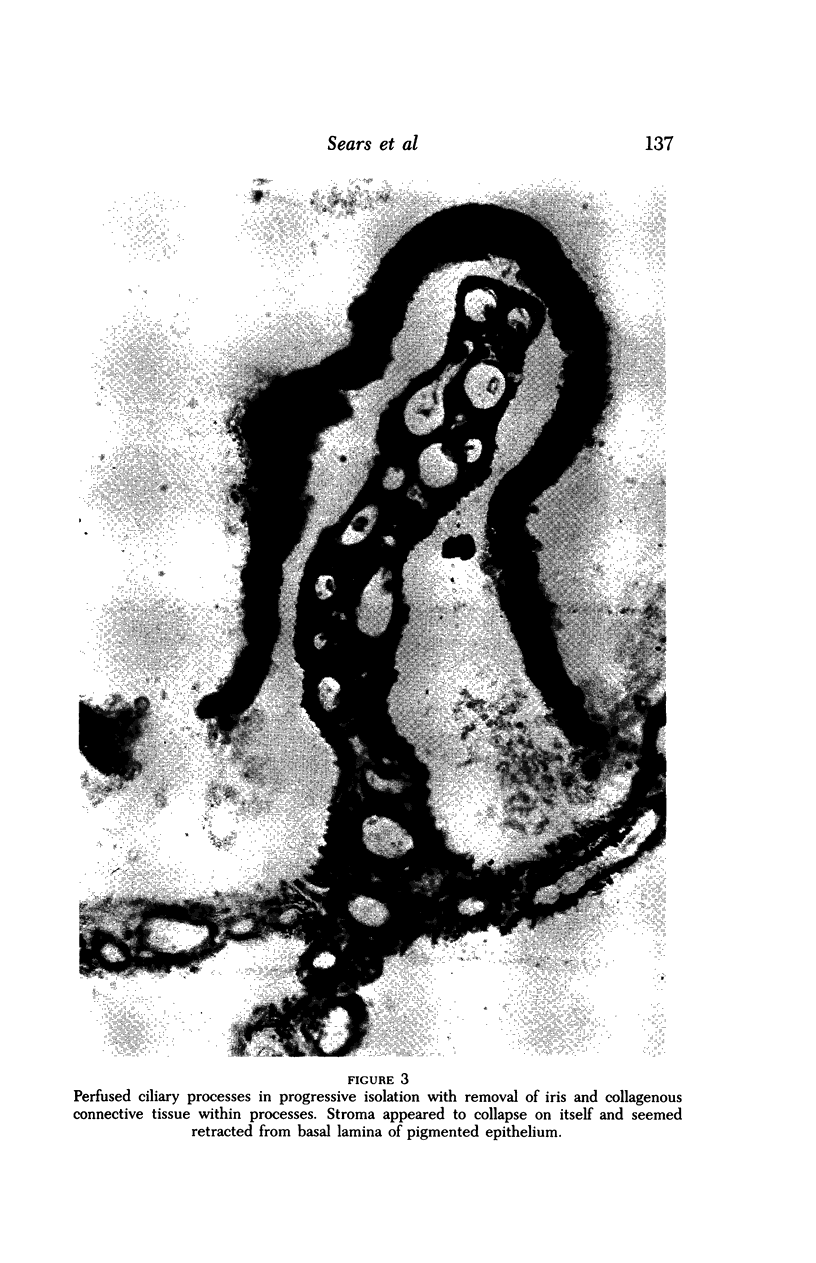
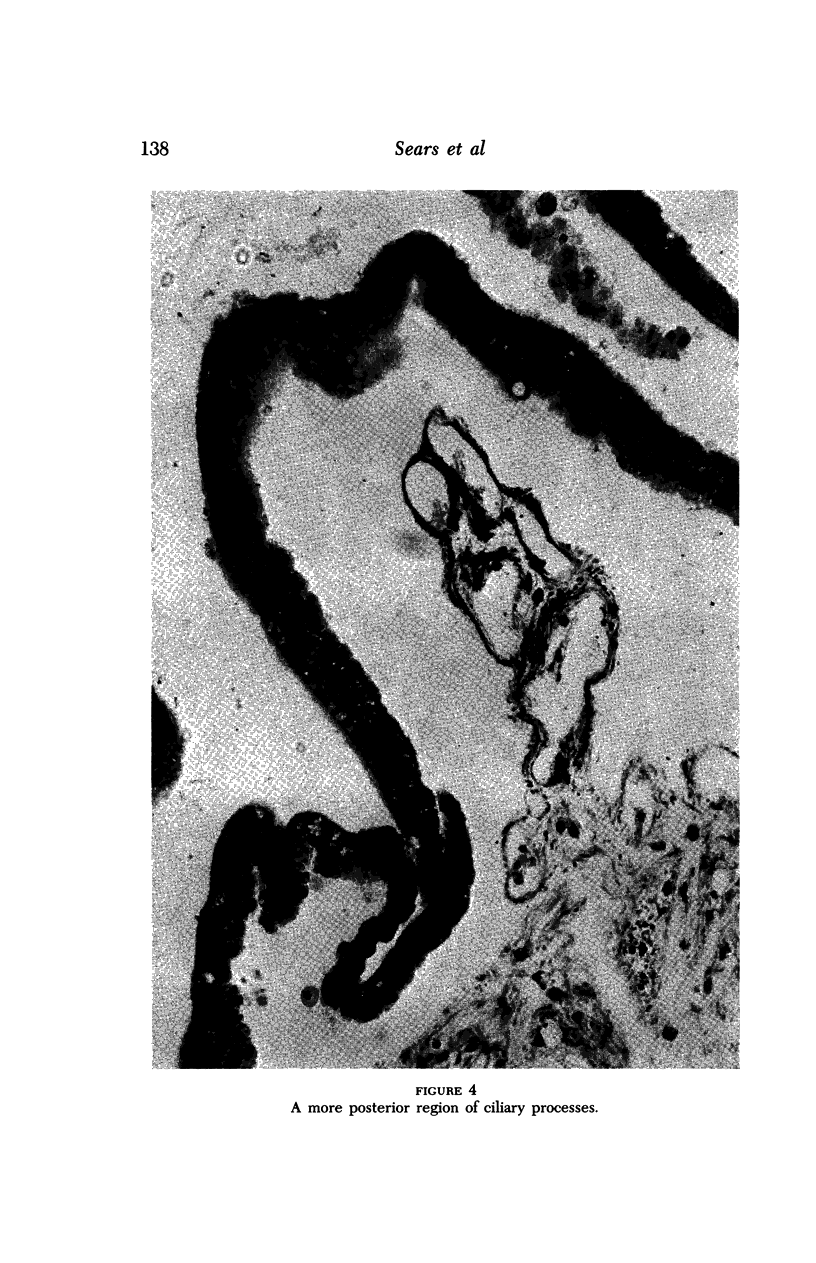
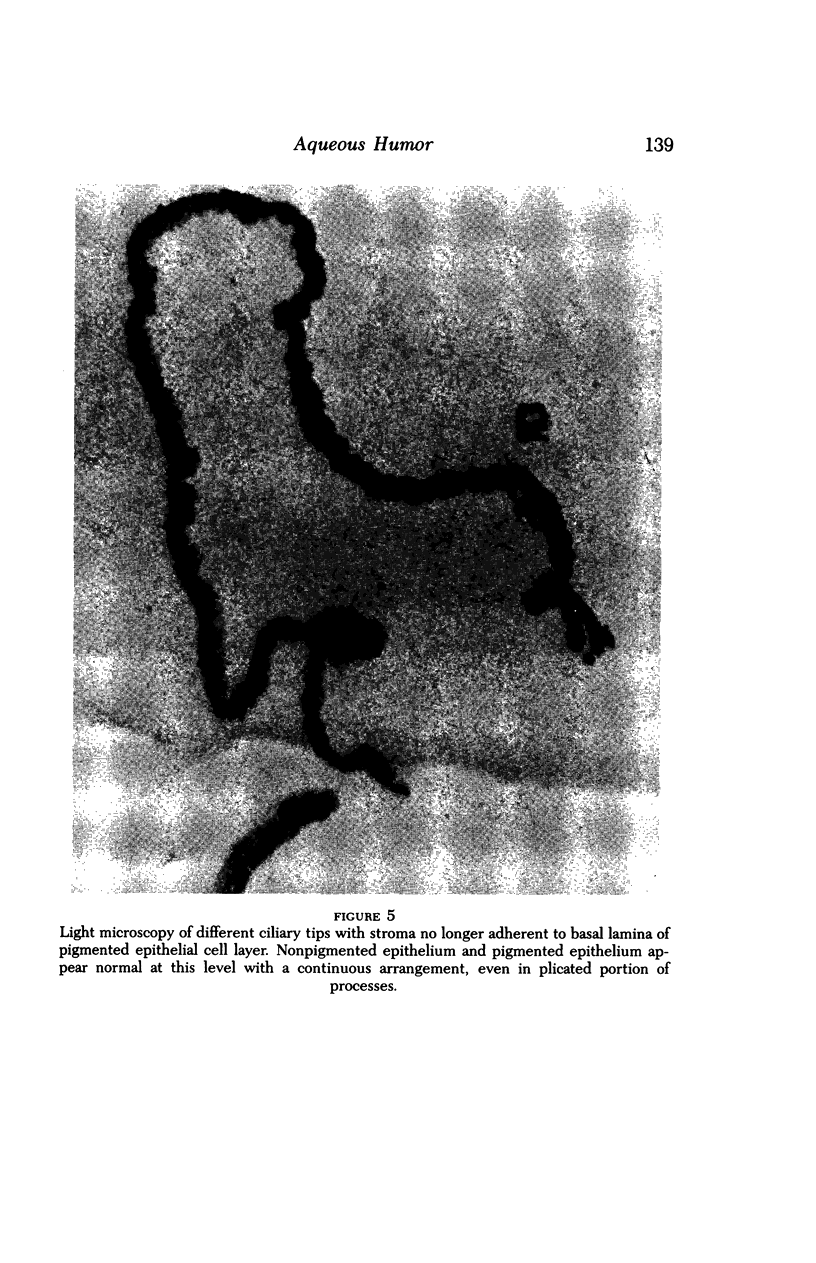
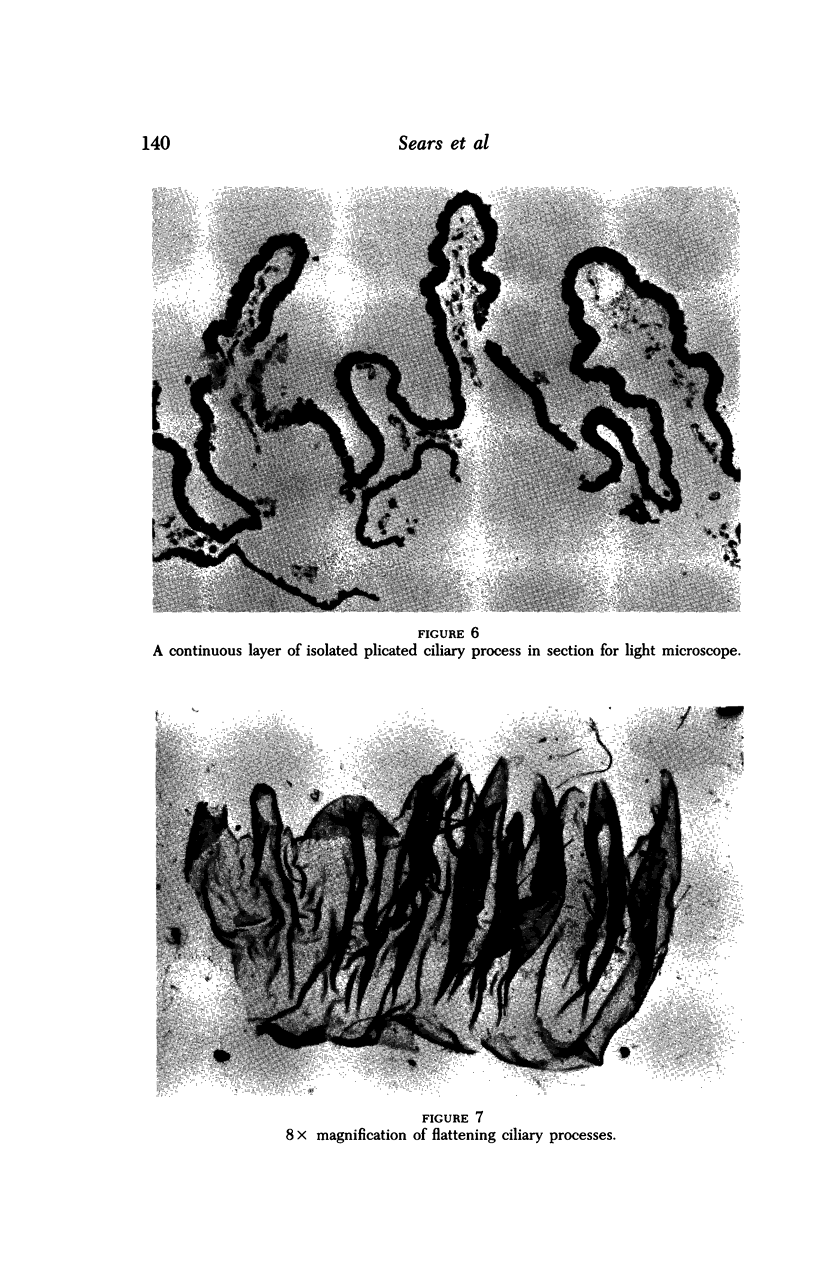



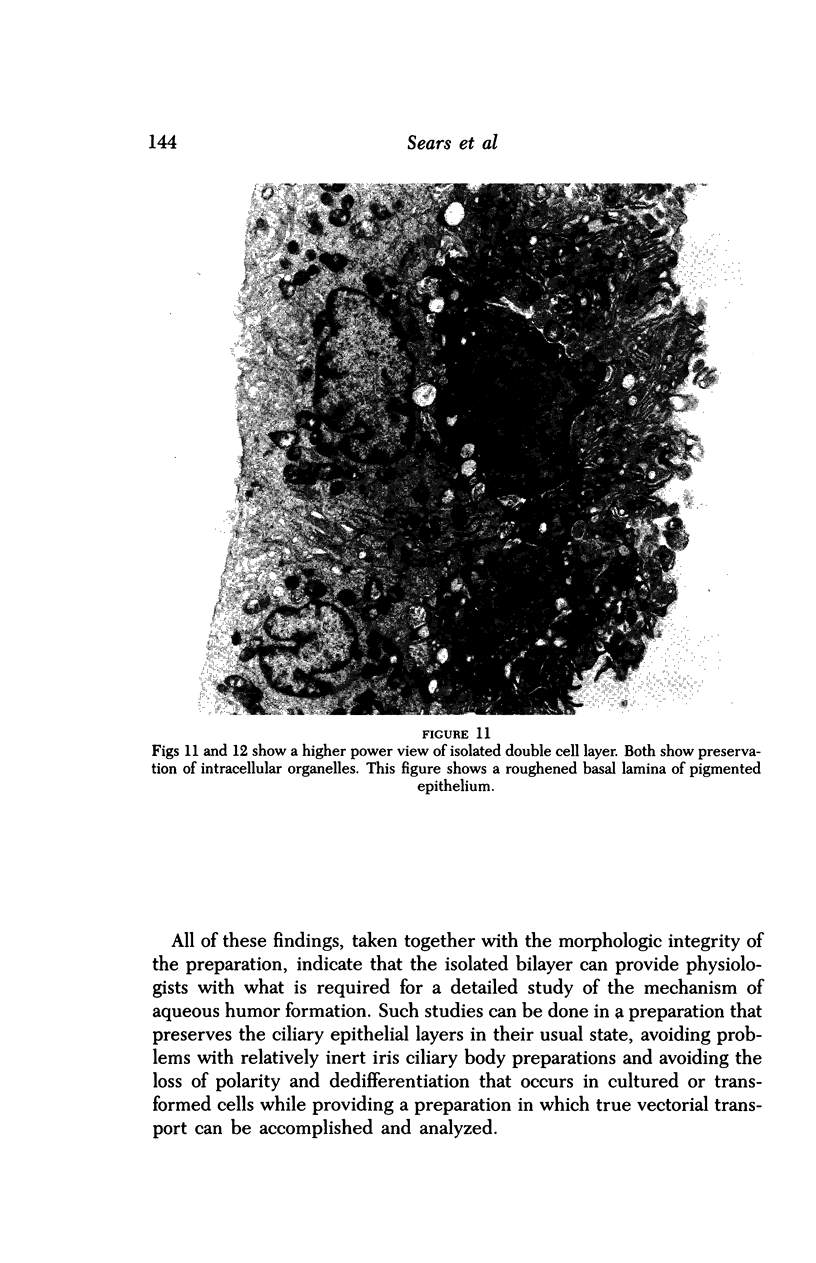
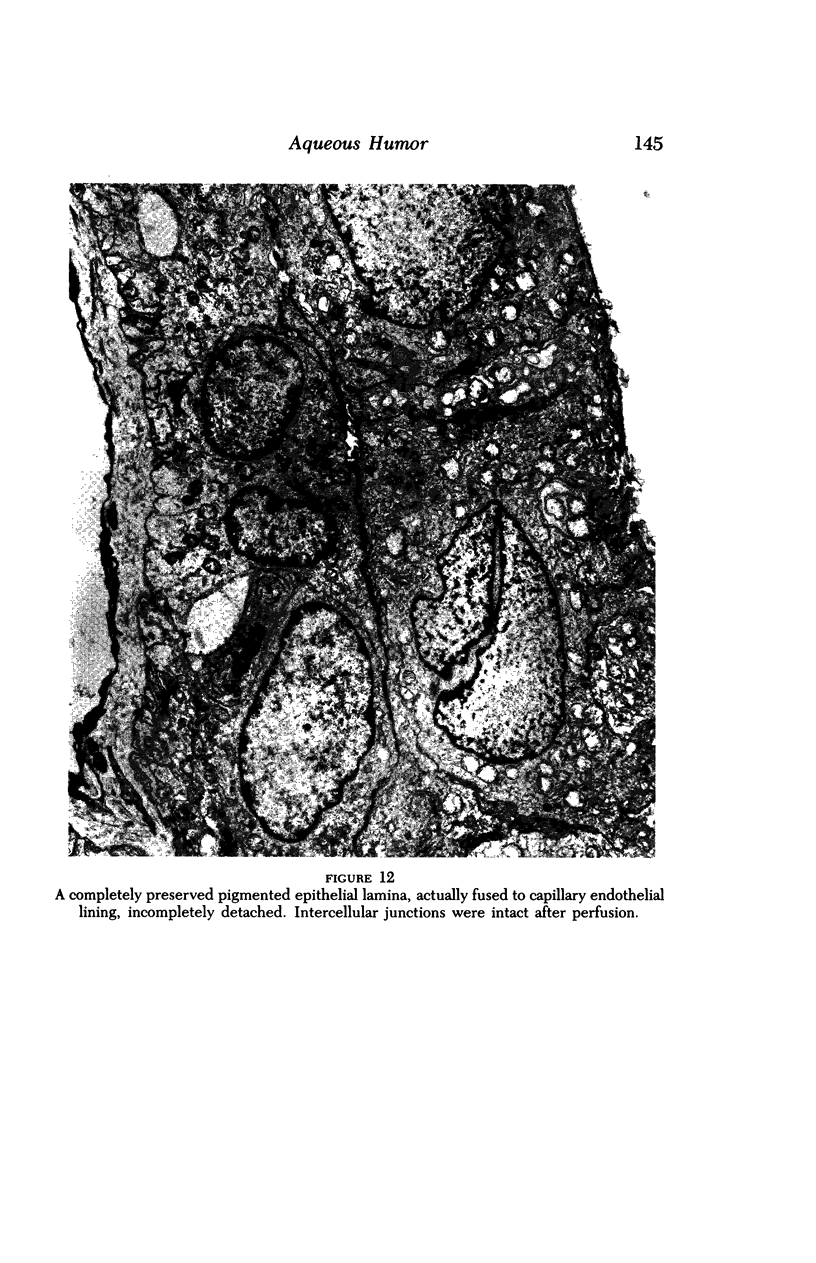
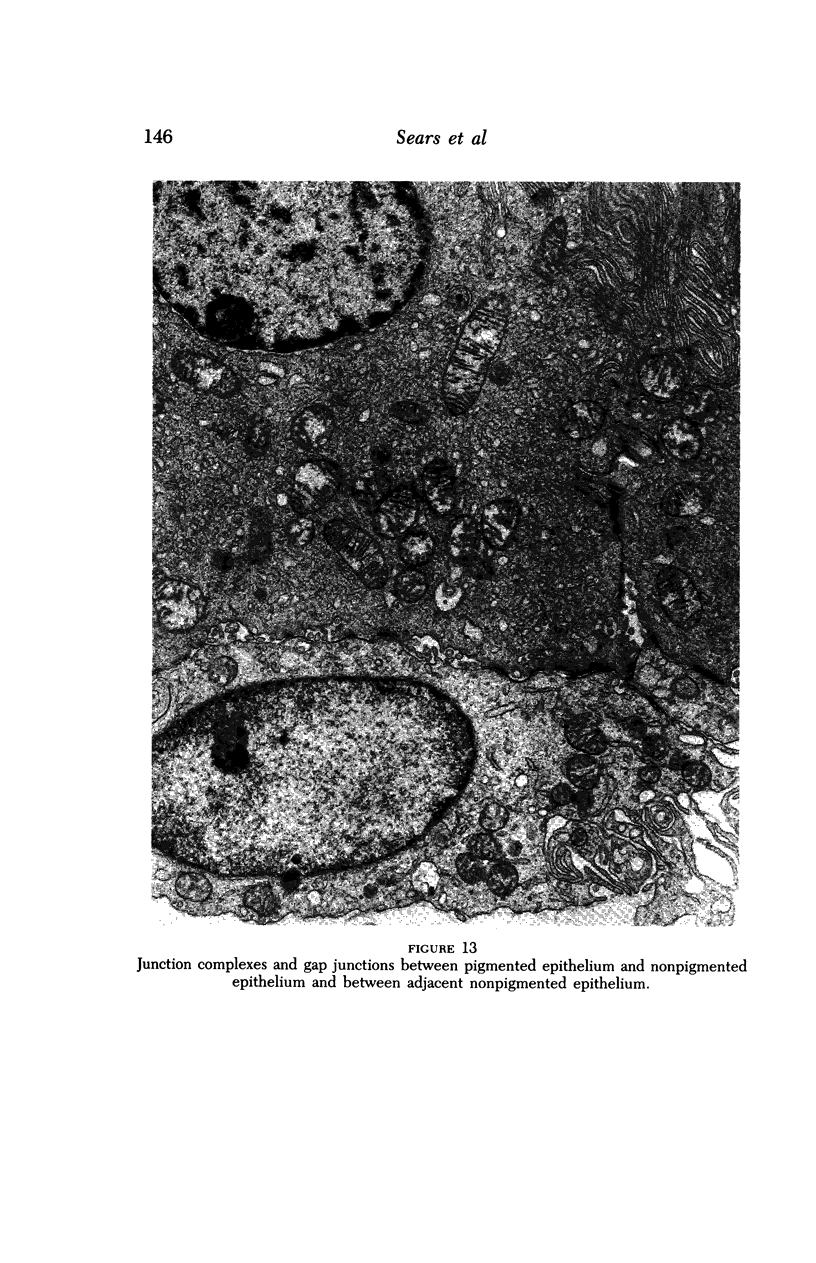
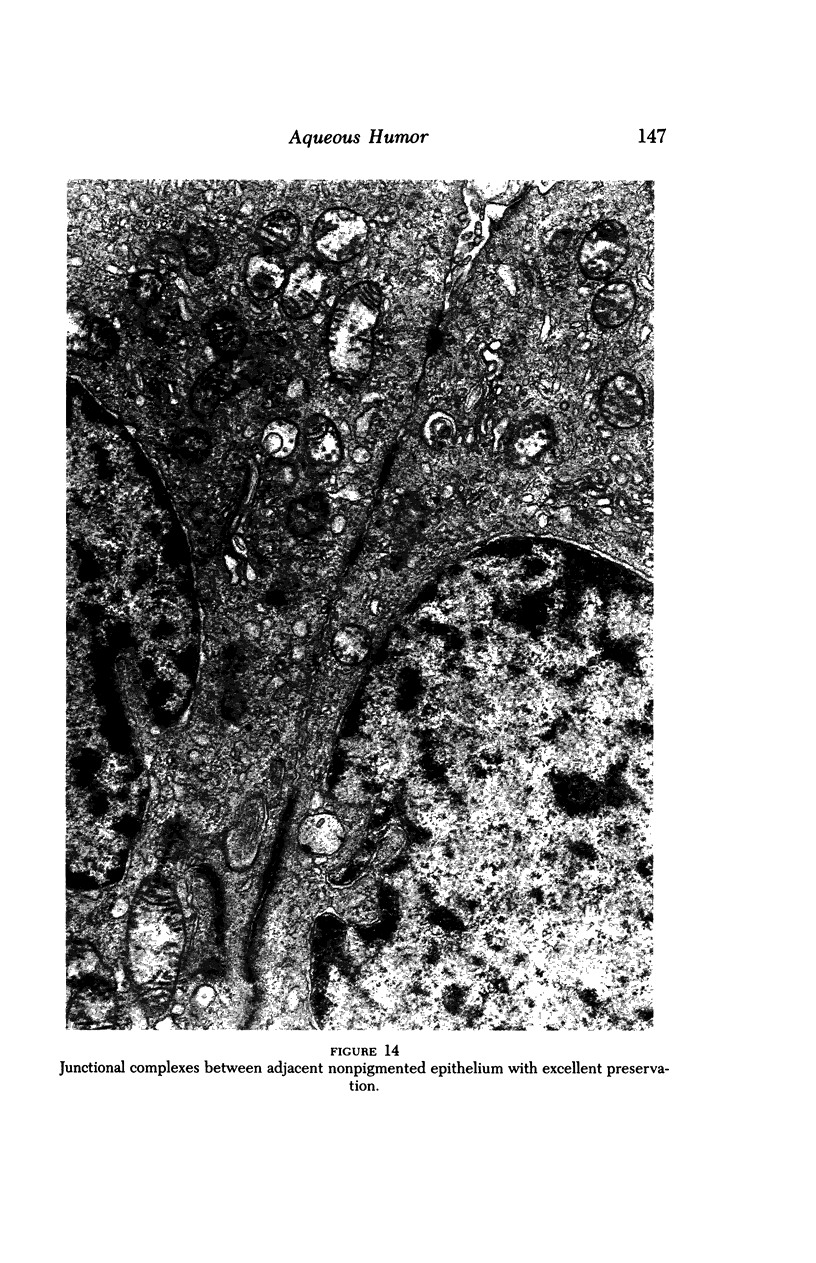



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu T. C., Candia O. A. Active transport of ascorbate across the isolated rabbit ciliary epithelium. Invest Ophthalmol Vis Sci. 1988 Apr;29(4):594–599. [PubMed] [Google Scholar]
- Coca-Prados M., Chatt G. Growth of non-pigmented ciliary epithelial cells in serum-free hormone-supplemented media. Exp Eye Res. 1986 Oct;43(4):617–629. doi: 10.1016/s0014-4835(86)80028-8. [DOI] [PubMed] [Google Scholar]
- Kishida K., Sasabe T., Iizuka S., Manabe R., Otori T. Sodium and chloride transport across the isolated rabbit ciliary body. Curr Eye Res. 1982;2(3):149–152. doi: 10.3109/02713688208997688. [DOI] [PubMed] [Google Scholar]
- Kondo K., Coca-Prados M., Sears M. Human ciliary epithelia in monolayer culture. Exp Eye Res. 1984 Apr;38(4):423–433. doi: 10.1016/0014-4835(84)90197-0. [DOI] [PubMed] [Google Scholar]
- Krampen G., von Eye A. Generalized expectations of drug-delinquents, other delinquents, and a control sample. Addict Behav. 1984;9(4):421–423. doi: 10.1016/0306-4603(84)90046-7. [DOI] [PubMed] [Google Scholar]
- Krupin T., Reinach P. S., Candia O. A., Podos S. M. Transepithelial electrical measurements on the isolated rabbit iris-ciliary body. Exp Eye Res. 1984 Feb;38(2):115–123. doi: 10.1016/0014-4835(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Flügel C., Lütjen-Drecoll E., Zadunaisky J. A. Mechanically stripped pigmented and non-pigmented epithelium of the shark ciliary body: morphology and transepithelial electrical properties. Exp Eye Res. 1989 Dec;49(6):1031–1043. doi: 10.1016/s0014-4835(89)80024-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. J., Garg L. C., Vogh B. P., Maren T. H. The effect of acetazolamide on the movement of sodium into the posterior chamber of the dog eye. J Pharmacol Exp Ther. 1976 Dec;199(3):510–517. [PubMed] [Google Scholar]