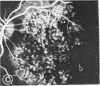
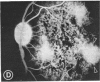
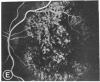
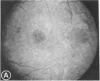
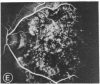
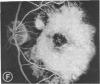
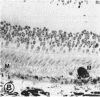
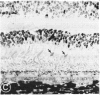
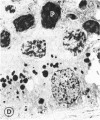
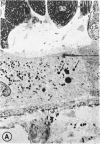
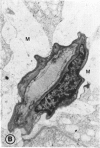
Abstract
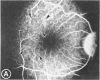
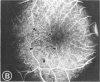
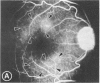
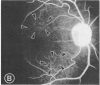
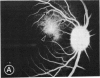
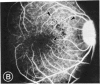
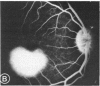
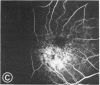
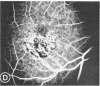
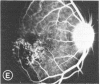
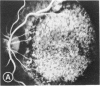
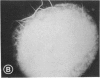
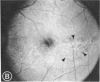
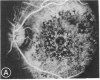
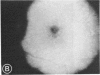
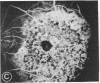
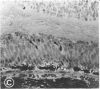
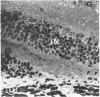
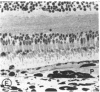
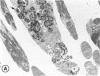
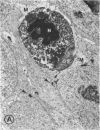
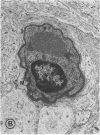
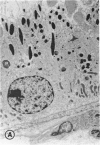
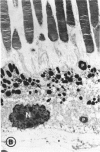
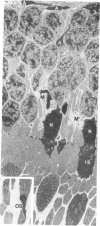
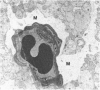
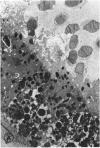
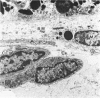
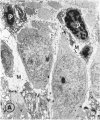
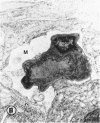
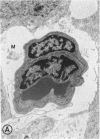
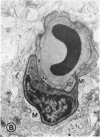
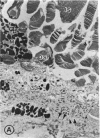
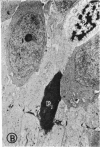
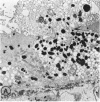
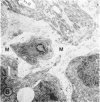
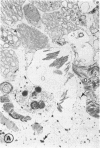
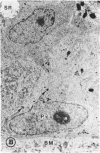
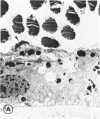
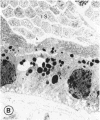
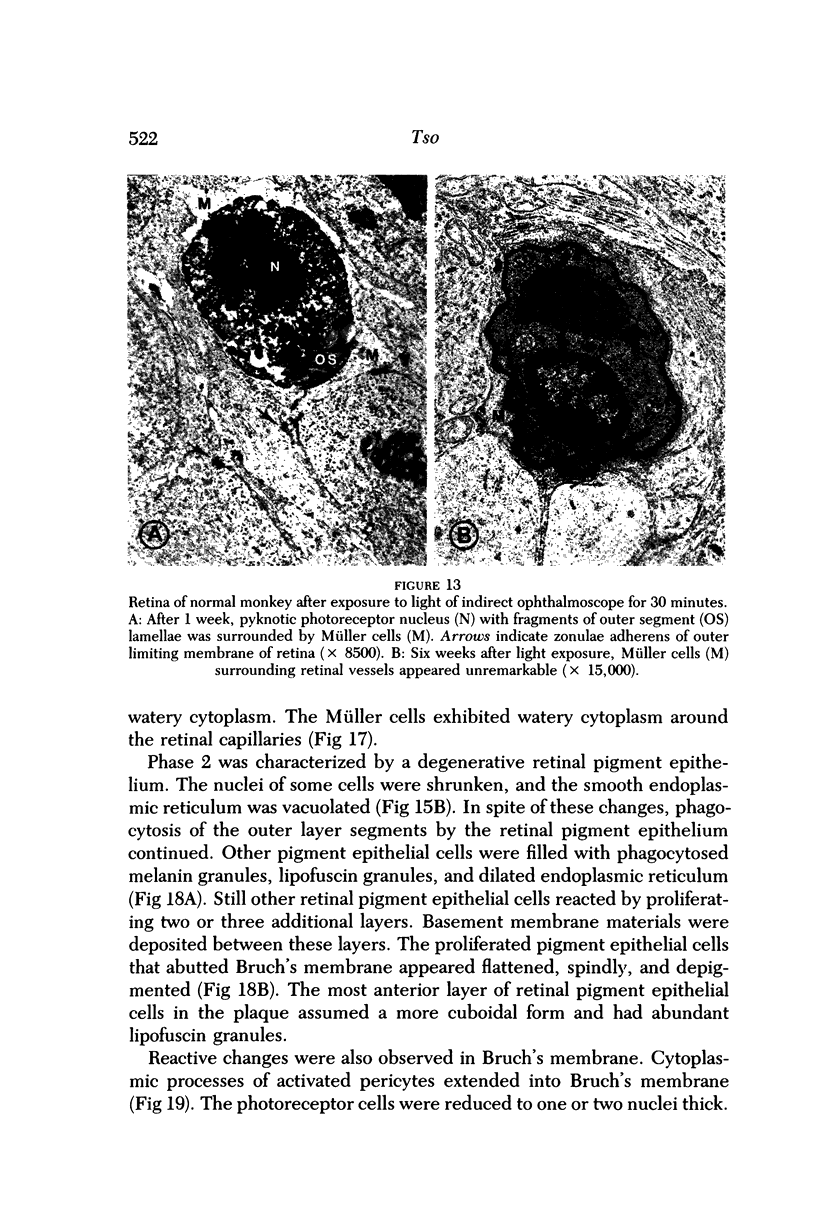
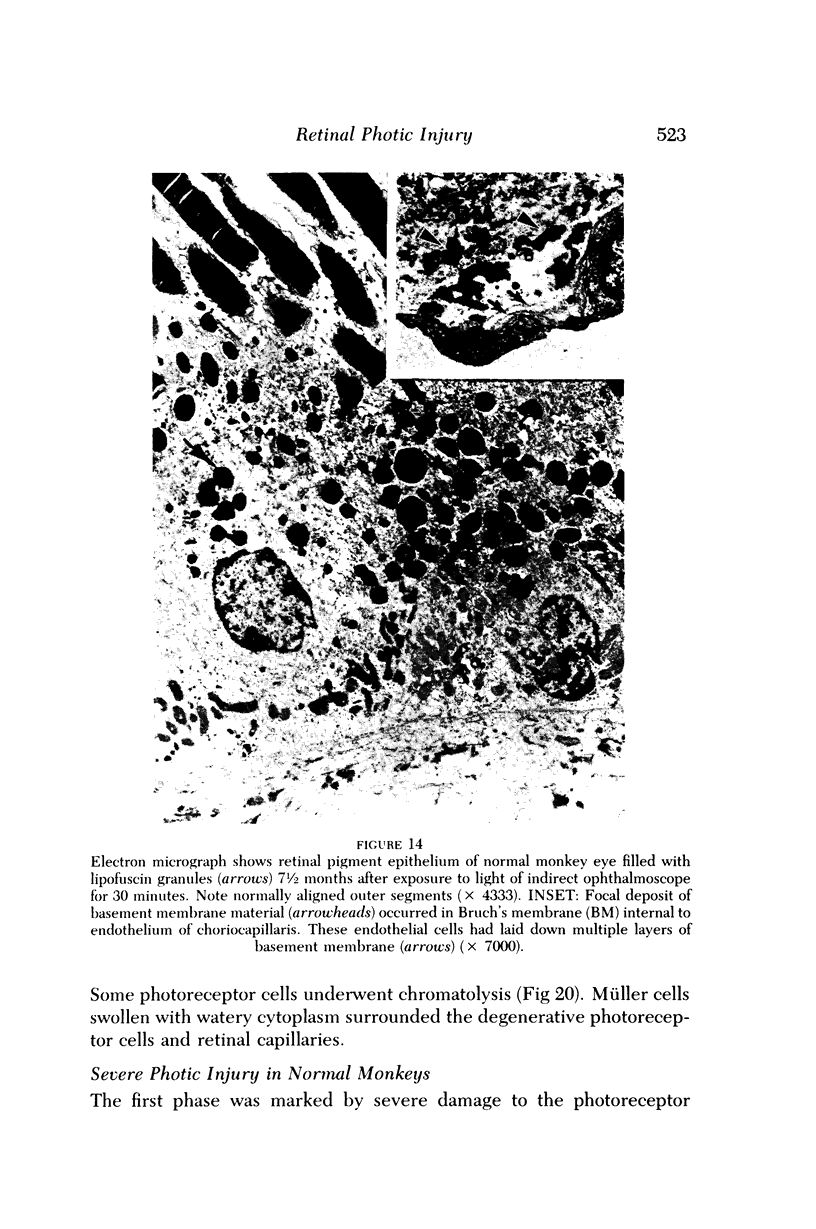
Mild and severe retinal photic injuries were inflicted on 22 eyes of seven monkeys fed a vitamin C-deficient diet and four monkeys given a vitamin C-enriched diet. The retinal lesions were studied by fundus examination, fluorescein angiography, and light and electron microscopy. While the general cellular response to photic injury in the retina of scorbutic animals was not different qualitatively from that in the normal animals, scurvy appeared to cause more severe tissue damage, an exaggerated repair response, and more advanced retinal degeneration. In the four groups of eyes, representing mild and severe photic injury in normal and scorbutic animals, a continuous spectrum of changes was produced. The least damage occurred from mild photic injury in the normal animals, and the most detrimental insult resulted from severe photic injury in the scorbutic animals. We propose that the basic mechanism by which ascorbate mitigates retinal photic injury depends on its redox properties. Ascorbate functions as an antioxidant in the retina. It scavenges superoxide radicals and hydroxyl radicals, quenches singlet oxygen, and reduces hydrogen peroxide, all of which are formed in retinal photic injury. This hypothesis provides an explanation for the high level of ascorbate in the retina. The pathogenetic mechanisms that correspond to the three distinct phases of pathologic changes observed in retinal photic injury are characterized. In phase 1, single oxygen is generated in a photodynamic reaction that damages the photoreceptor elements and pigment epithelium. In phase 2, macrophages attracted from the systemic circulation invade the subretinal space, and a photo-oxidative reaction generates superoxide radicals, hydrogen peroxide, and hydroxyl radicals. These free radicals attack the photoreceptor cells and pigment epithelium to cause further retinal damage. In phase 3, macrophages remain in the subretinal space for as long as 8 months after injury, causing persistent disruption of the blood-retinal barrier. The photo-oxidative reaction appears to linger, resulting in chronic retinal degeneration. It is hypothesized that in some forms of age-related macular degeneration, patients suffer from repeated mild photic insult throughout their lifetime. Aging has been associated with subclinical scurvy, which leads to even greater susceptibility to photic injury. Although ascorbate moderates many biochemical functions of the body and helps the retina ameliorate photo-oxidative injury, it should be regarded as a nutritional supplement to maintain health when consumed in appropriate amounts and not as a therapeutic agent for the treatment of severe insults.
Full text
PDF









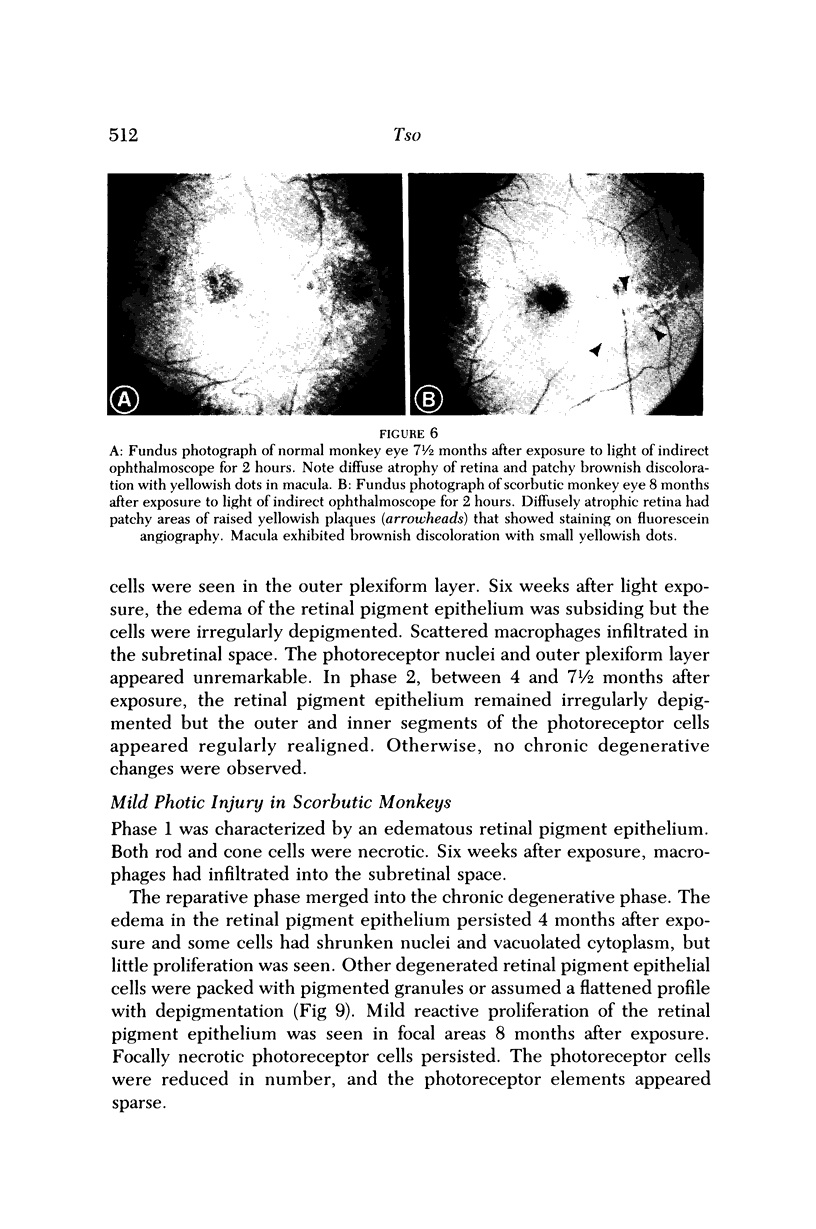
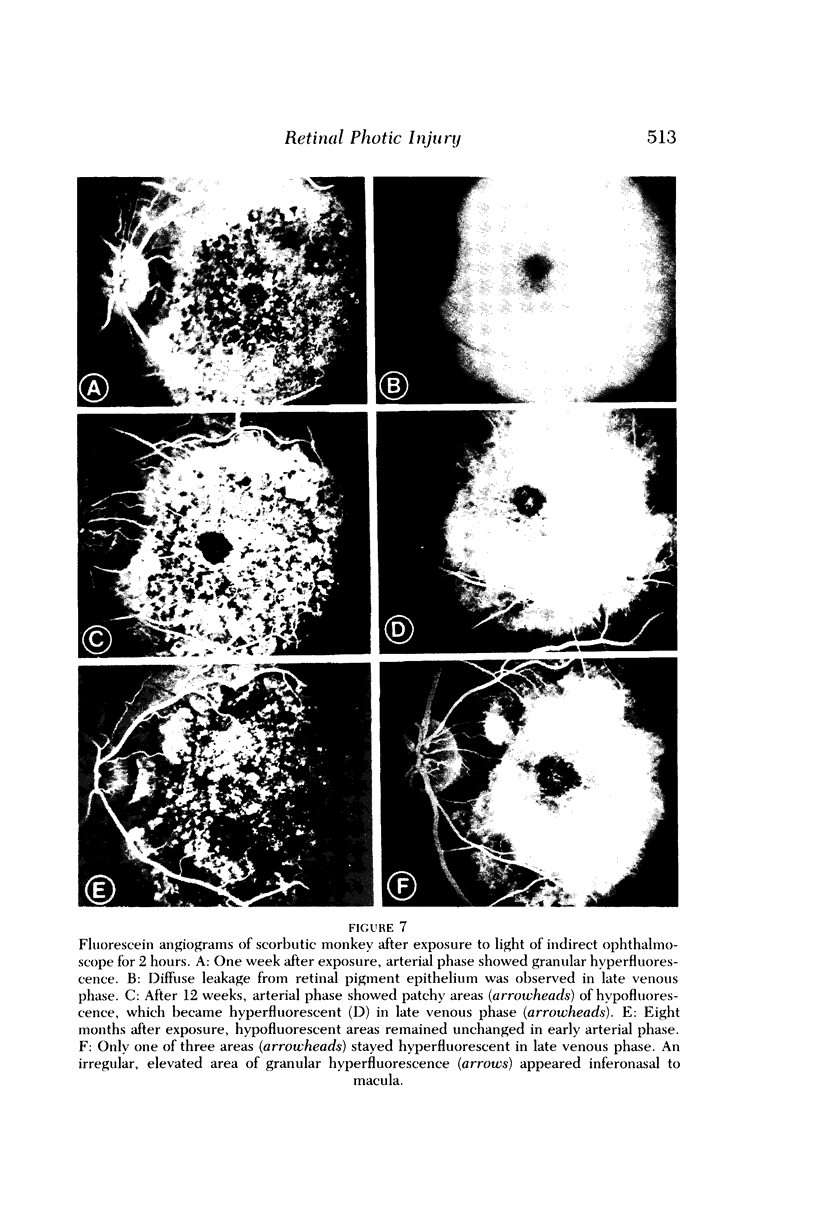
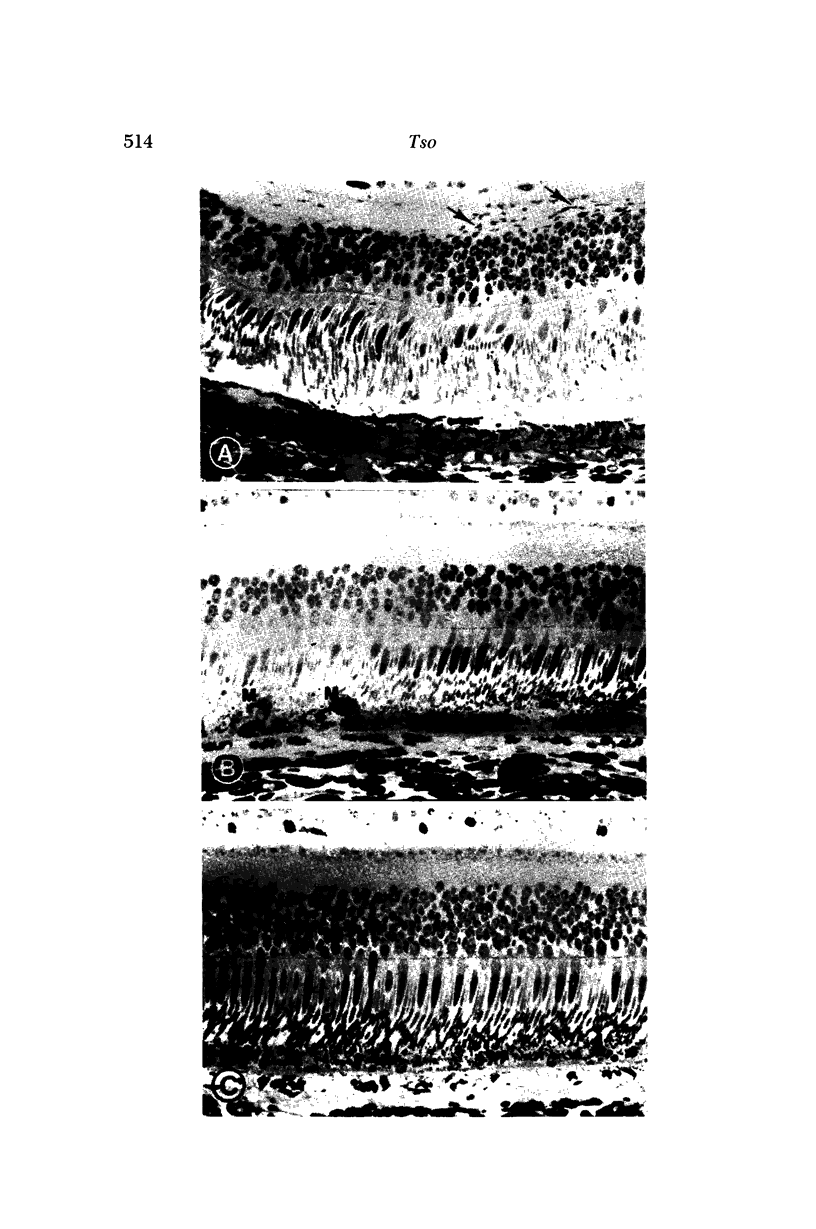

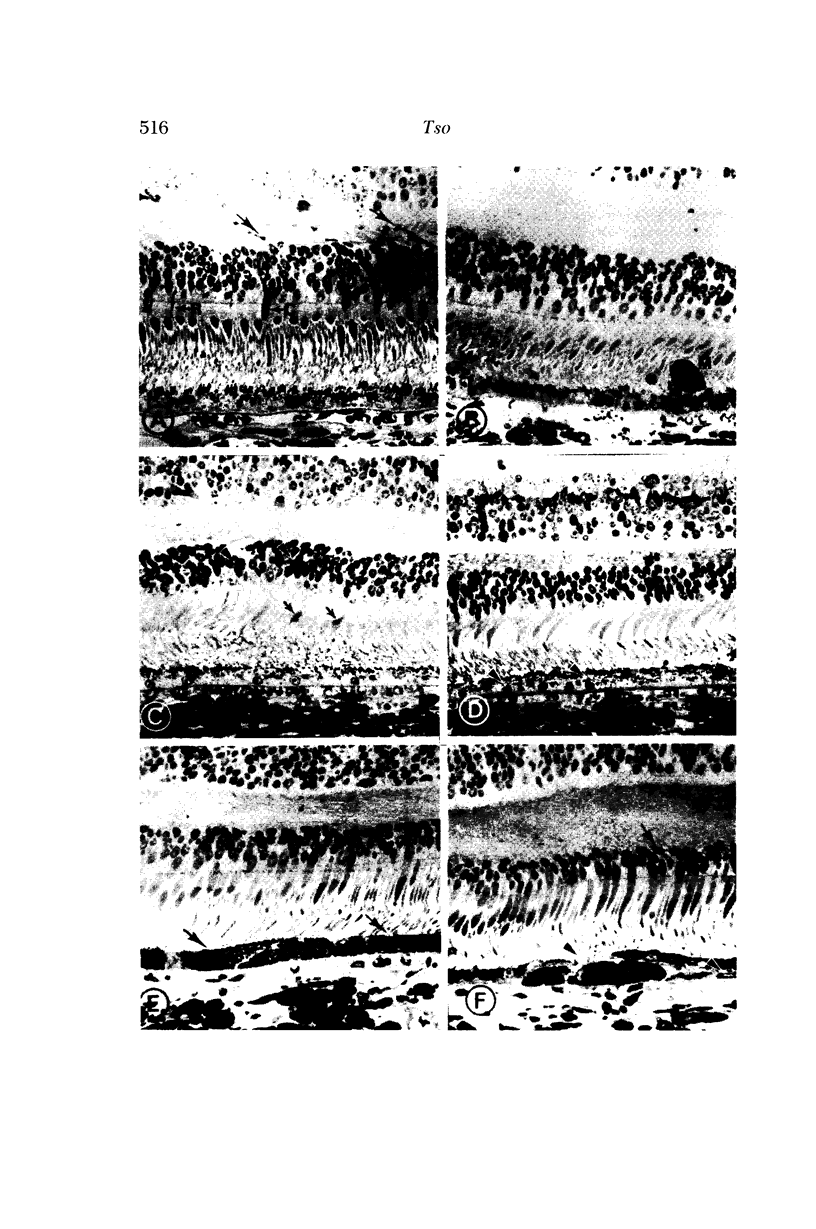






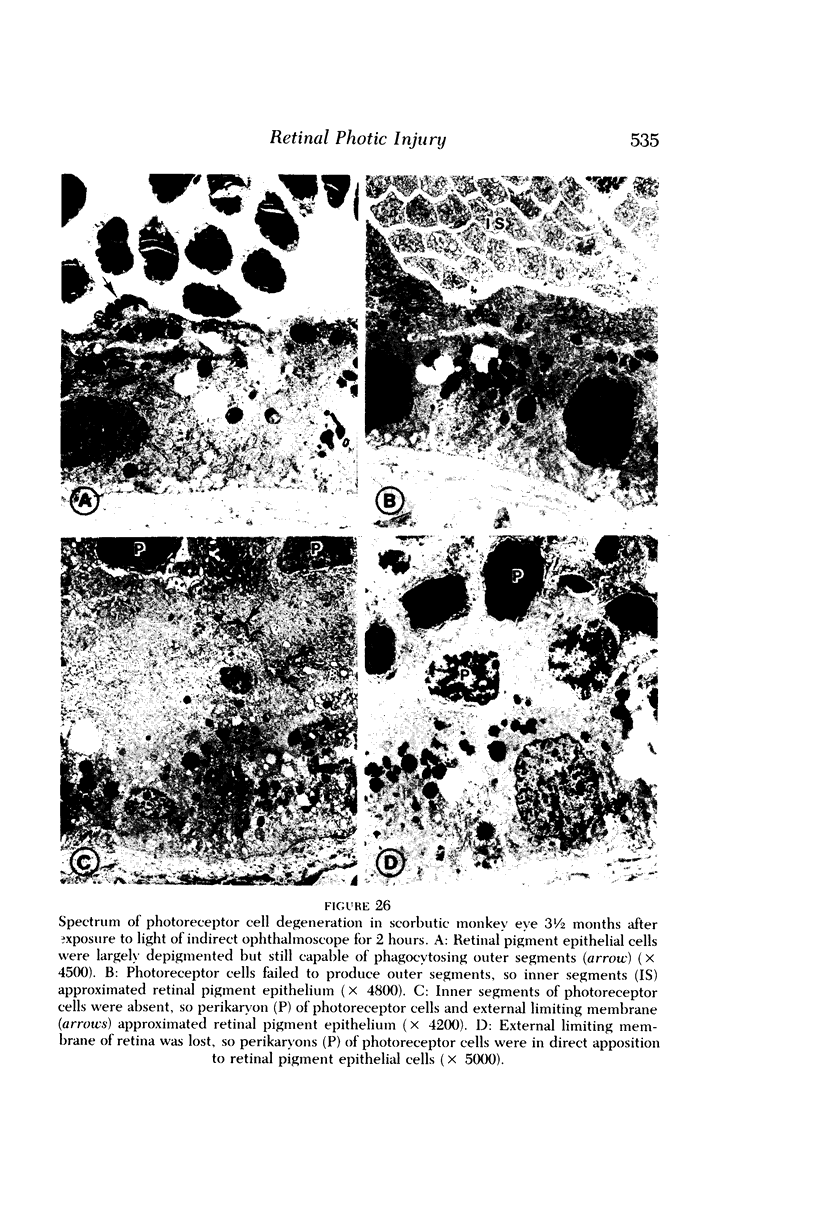
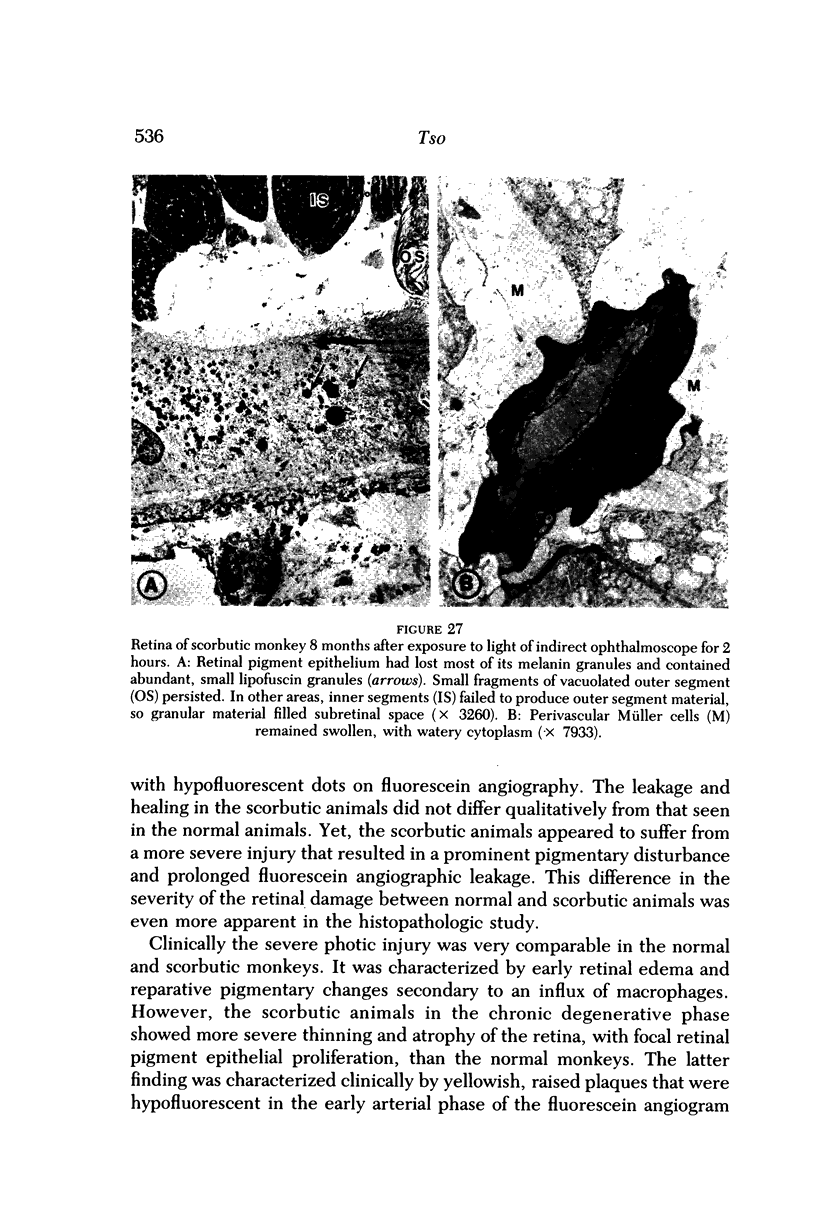







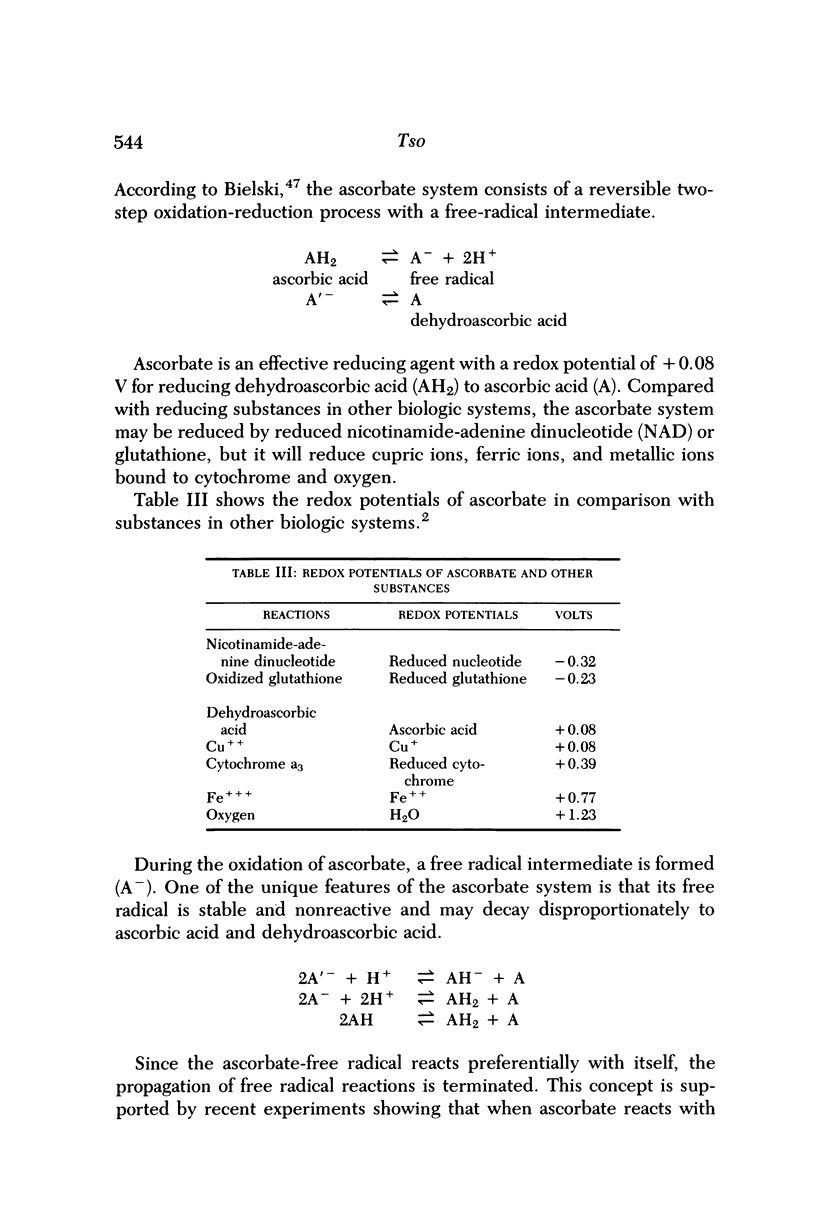












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Maude M. B., Nielsen J. C. Effect of lipid peroxidation on rhodopsin regeneration. Curr Eye Res. 1985 Jan;4(1):65–71. doi: 10.3109/02713688508999969. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Rapp L. M., Wiegand R. D. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984 Jan;3(1):223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- BECKER B., LINNER E. Ascorbic acid as a test substance for measuring relative changes in the rate of plasma flow through the ciliary processes. III. The effect of pre-ganglionic section of the cervical sympathetic in rabbits on the ascorbic acid content of the aqueous humour at varying plasma levels. Acta Physiol Scand. 1952 Jul 17;26(1):79–85. doi: 10.1111/j.1748-1716.1952.tb00893.x. [DOI] [PubMed] [Google Scholar]
- BOYD T. A. Influence of local ascorbic acid concentration on collagenous tissue healing in the cornea. Br J Ophthalmol. 1955 Apr;39(4):204–214. doi: 10.1136/bjo.39.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E. M., Hodges R. E., Hood J., Sauberlich H. E., March S. C., Canham J. E. Metabolism of 14C- and 3H-labeled L-ascorbic acid in human scurvy. Am J Clin Nutr. 1971 Apr;24(4):444–454. doi: 10.1093/ajcn/24.4.444. [DOI] [PubMed] [Google Scholar]
- Baker E. M., Saari J. C., Tolbert B. M. Ascorbic acid metabolism in man. Am J Clin Nutr. 1966 Dec;19(6):371–378. doi: 10.1093/ajcn/19.5.371. [DOI] [PubMed] [Google Scholar]
- Bhuyan K. C., Bhuyan D. K. Molecular mechanism of cataractogenesis: III. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Curr Eye Res. 1984 Jan;3(1):67–81. doi: 10.3109/02713688408997188. [DOI] [PubMed] [Google Scholar]
- Bodannes R. S., Chan P. C. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979 Sep 15;105(2):195–196. doi: 10.1016/0014-5793(79)80609-2. [DOI] [PubMed] [Google Scholar]
- Boldrey E. E., Ho B. T., Griffith R. D. Retinal burns occurring at cataract extraction. Ophthalmology. 1984 Nov;91(11):1297–1302. doi: 10.1016/s0161-6420(84)34150-1. [DOI] [PubMed] [Google Scholar]
- CRISTIANSSON J. Changes in the vitreous body in scurvy; studies on guinea pigs in vivo. I. The biopolymers of the vitreous body. Acta Ophthalmol (Copenh) 1957;35(4):336–360. doi: 10.1111/j.1755-3768.1957.tb07696.x. [DOI] [PubMed] [Google Scholar]
- Calkins J. L., Hochheimer B. F., D'Anna S. A. Potential hazards from specific ophthalmic devices. Vision Res. 1980;20(12):1039–1053. doi: 10.1016/0042-6989(80)90042-5. [DOI] [PubMed] [Google Scholar]
- DAS T., NIRANKARI M. S., CHADDAH M. R. Solar chorioretinal burn. Am J Ophthalmol. 1956 Jun;41(6):1048–1053. doi: 10.1016/0002-9394(56)91056-x. [DOI] [PubMed] [Google Scholar]
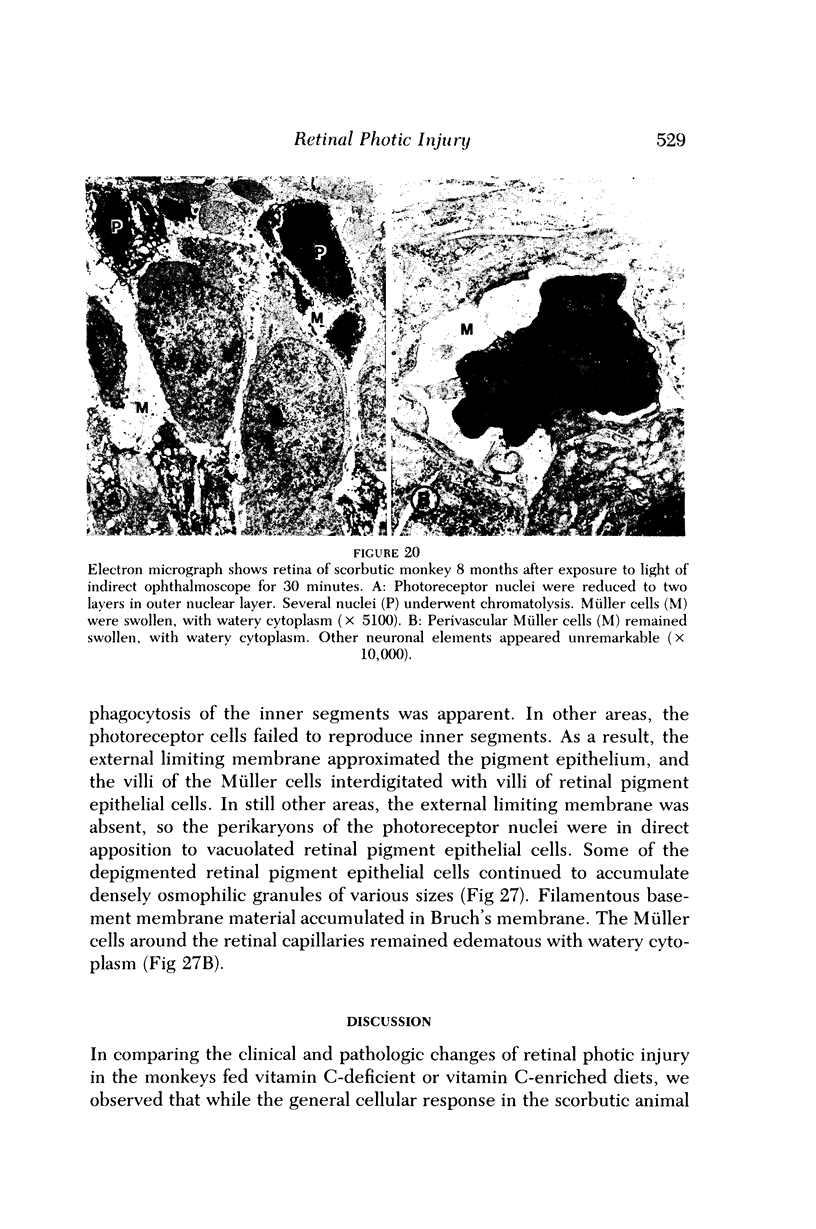
- Delmelle M. Possible implication of photooxidation reactions in retinal photo-damage. Photochem Photobiol. 1979 Apr;29(4):713–716. doi: 10.1111/j.1751-1097.1979.tb07755.x. [DOI] [PubMed] [Google Scholar]
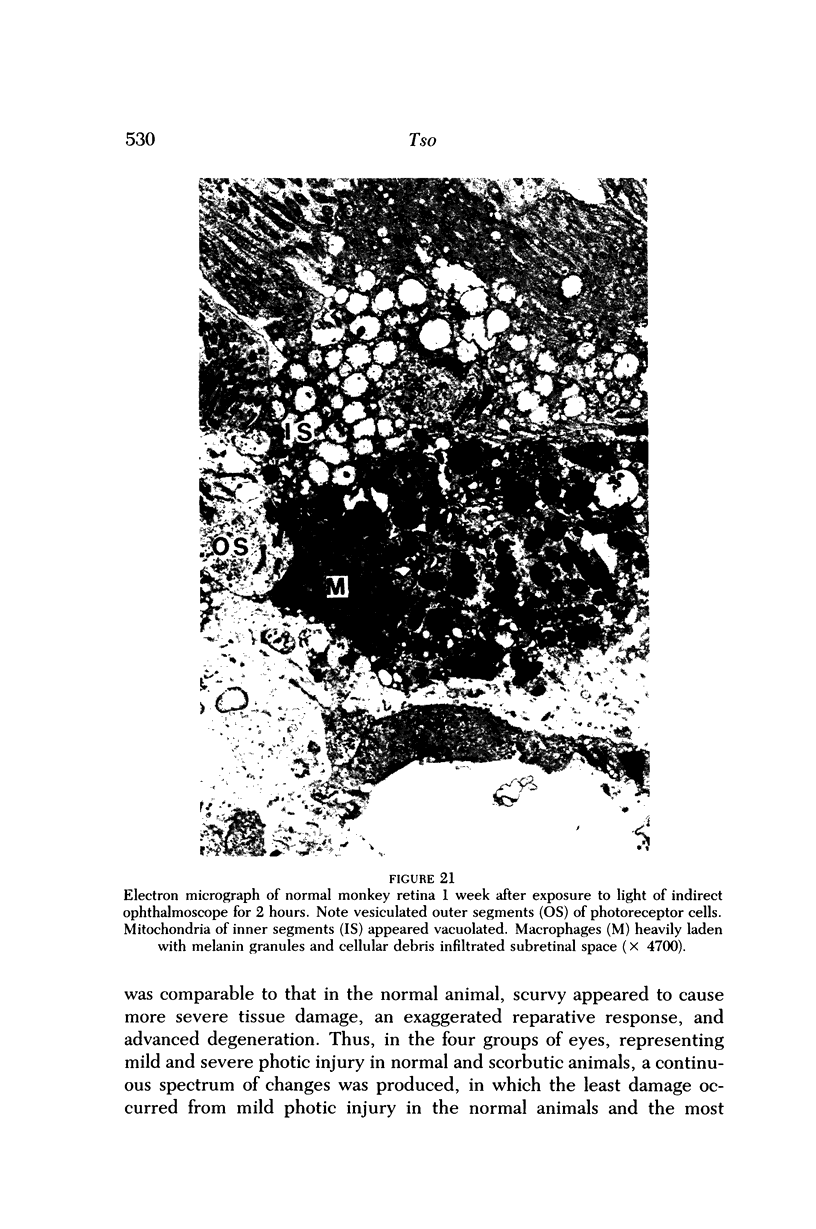
- Duvall J., Tso M. O. Cellular mechanisms of resolution of drusen after laser coagulation. An experimental study. Arch Ophthalmol. 1985 May;103(5):694–703. doi: 10.1001/archopht.1985.01050050086024. [DOI] [PubMed] [Google Scholar]
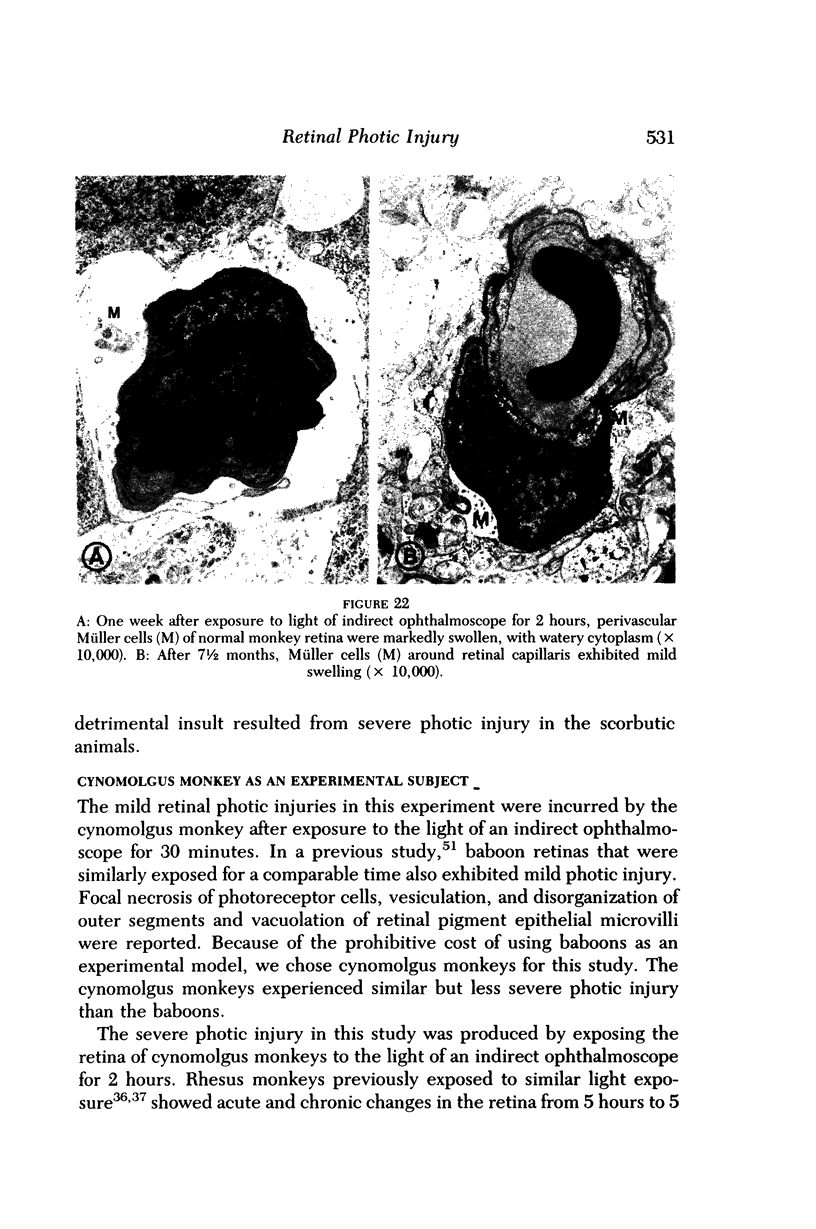
- Eigner E. H. Self-induced solar retinitis. Am J Ophthalmol. 1966 Jun;61(6):1546–1547. doi: 10.1016/0002-9394(66)90509-5. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L., Berman E. R., Rothman H. Lipofuscin of human retinal pigment epithelium. Am J Ophthalmol. 1980 Dec;90(6):783–791. doi: 10.1016/s0002-9394(14)75193-1. [DOI] [PubMed] [Google Scholar]
- Feeney L., Berman E. R. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol. 1976 Oct;15(10):789–792. [PubMed] [Google Scholar]
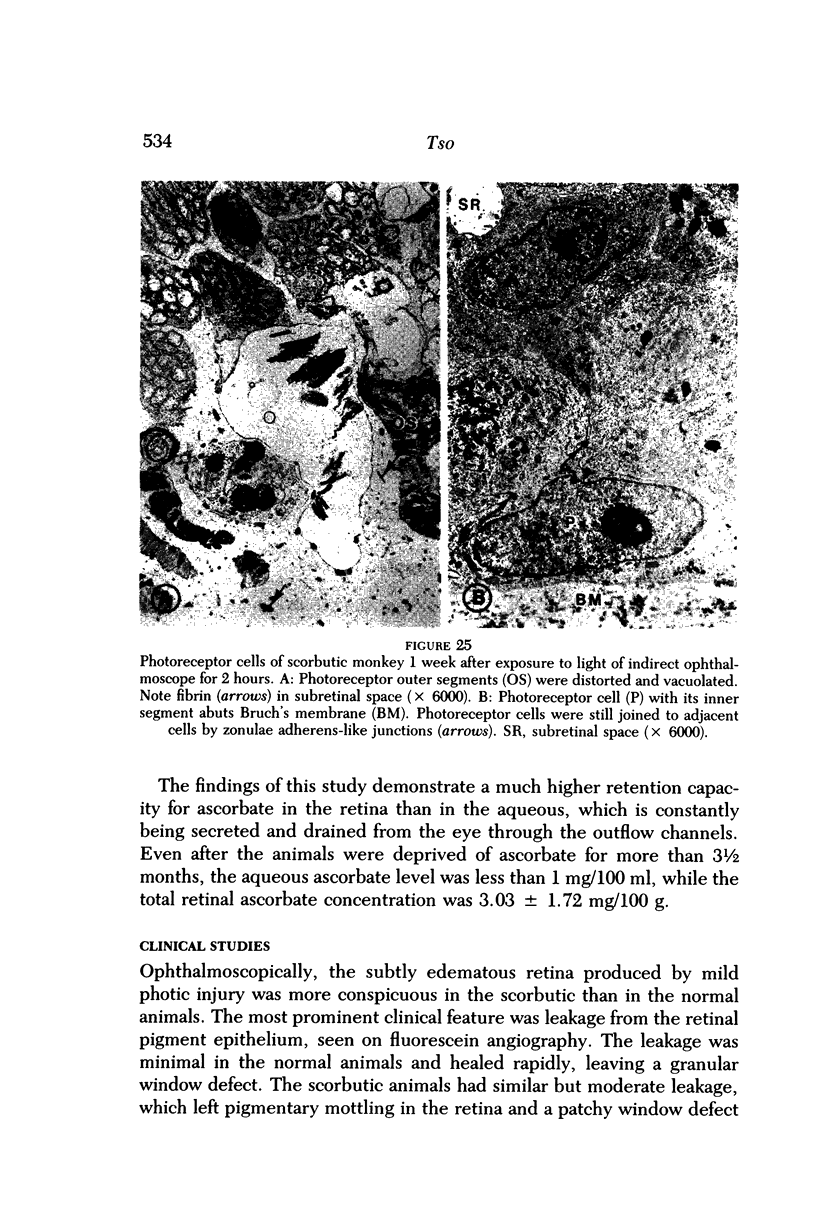
- Fiddick R., Heath H. The in vivo uptake of l-[1-14-C]ascorbic acid by the rat retina and adrenal gland. Exp Eye Res. 1966 Oct;5(4):329–334. doi: 10.1016/s0014-4835(66)80044-1. [DOI] [PubMed] [Google Scholar]
- Fine B. S., Brucker A. J. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981 Oct;92(4):466–481. doi: 10.1016/0002-9394(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Fox R. R., Lam K. W., Lewen R., Lee P. Ascorbate concentration in tissues from normal and buphthalmic rabbits. J Hered. 1982 Mar-Apr;73(2):109–111. doi: 10.1093/oxfordjournals.jhered.a109590. [DOI] [PubMed] [Google Scholar]
- Friedenwald J. S., Buschke W., Michel H. O. The Rôle of Ascorbic Acid (Vitamin C) in the Secretion of the Intra-Ocular Fluid. Trans Am Ophthalmol Soc. 1939;37:310–335. [PMC free article] [PubMed] [Google Scholar]
- Friedman E., Kuwabara T. The retinal pigment epithelium. IV. The damaging effects of radiant energy. Arch Ophthalmol. 1968 Aug;80(2):265–279. doi: 10.1001/archopht.1968.00980050267022. [DOI] [PubMed] [Google Scholar]
- Fuller D., Machemer R., Knighton R. W. Retinal damage produced by intraocular fiber optic light. Am J Ophthalmol. 1978 Apr;85(4):519–537. doi: 10.1016/s0002-9394(14)75250-x. [DOI] [PubMed] [Google Scholar]
- Gartner S., Henkind P. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br J Ophthalmol. 1981 Jan;65(1):23–28. doi: 10.1136/bjo.65.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J. D., Anderson D. R., Davis E. B. A clinical, fluorescein angiographic, and electron microscopic correlation of cystoid macular edema. Am J Ophthalmol. 1985 Jul 15;100(1):82–86. doi: 10.1016/s0002-9394(14)74988-8. [DOI] [PubMed] [Google Scholar]
- Gladstone G. J., Tasman W. Solar retinitis after minimal exposure. Arch Ophthalmol. 1978 Aug;96(8):1368–1369. doi: 10.1001/archopht.1978.03910060122004. [DOI] [PubMed] [Google Scholar]
- Ham W. T., Jr, Mueller H. A., Ruffolo J. J., Jr, Clarke A. M. Sensitivity of the retina to radiation damage as a function of wavelength. Photochem Photobiol. 1979 Apr;29(4):735–743. doi: 10.1111/j.1751-1097.1979.tb07759.x. [DOI] [PubMed] [Google Scholar]
- Ham W. T., Jr, Mueller H. A., Sliney D. H. Retinal sensitivity to damage from short wavelength light. Nature. 1976 Mar 11;260(5547):153–155. doi: 10.1038/260153a0. [DOI] [PubMed] [Google Scholar]
- Ham W. T., Jr, Ruffolo J. J., Jr, Mueller H. A., Clarke A. M., Moon M. E. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest Ophthalmol Vis Sci. 1978 Oct;17(10):1029–1035. [PubMed] [Google Scholar]
- Harwerth R. S., Sperling H. G. Effects of intense visible radiation on the increment-threshold spectral sensitivity of the rhesus monkey eye. Vision Res. 1975 Nov;15(11):1193–1204. doi: 10.1016/0042-6989(75)90162-5. [DOI] [PubMed] [Google Scholar]
- Hayes K. C. Retinal degeneration in monkeys induced by deficiencies of vitamin E or A. Invest Ophthalmol. 1974 Jul;13(7):499–510. [PubMed] [Google Scholar]
- Henkes H. E. Photic injury to the retina and the manifestation of acute posterior multifocal placoid pigment epitheliopathy. Doc Ophthalmol. 1977 Sep 30;44(1):113–120. doi: 10.1007/BF00171462. [DOI] [PubMed] [Google Scholar]
- Henkes H. E. Photic injury to the retina and the manifestation of acute posterior multifocal placoid pigment epitheliopathy. Doc Ophthalmol. 1977 Sep 30;44(1):113–120. doi: 10.1007/BF00171462. [DOI] [PubMed] [Google Scholar]
- Howell W. L., Rapp L. M., Williams T. P. Distribution of melanosomes across the retinal pigment epithelium of a hooded rat: implications for light damage. Invest Ophthalmol Vis Sci. 1982 Feb;22(2):139–144. [PubMed] [Google Scholar]
- Hyman L. G., Lilienfeld A. M., Ferris F. L., 3rd, Fine S. L. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983 Aug;118(2):213–227. doi: 10.1093/oxfordjournals.aje.a113629. [DOI] [PubMed] [Google Scholar]
- Kallner A., Hartmann D., Hornig D. Steady-state turnover and body pool of ascorbic acid in man. Am J Clin Nutr. 1979 Mar;32(3):530–539. doi: 10.1093/ajcn/32.3.530. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Stone W. L., Dratz E. A. Fluorescent pigment accumulation in retinal pigment epithelium of antioxidant-deficient rats. Invest Ophthalmol Vis Sci. 1978 Nov;17(11):1049–1058. [PubMed] [Google Scholar]
- Klatzo I. Presidental address. Neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967 Jan;26(1):1–14. doi: 10.1097/00005072-196701000-00001. [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Gorn R. A. Retinal damage by visible light. An electron microscopic study. Arch Ophthalmol. 1968 Jan;79(1):69–78. doi: 10.1001/archopht.1968.03850040071019. [DOI] [PubMed] [Google Scholar]
- LINNER E. Ascorbic acid as a test substance for measuring relative changes in the rate of plasma flow through the ciliary processes. IV. The effect of carotid ligation and cervical sympathectomy in guinea-pigs on the ascorbic acid content of the aqueous humour at varying plasma levels. Acta Physiol Scand. 1952 Sep 10;26(2-3):130–147. doi: 10.1111/j.1748-1716.1952.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Lai Y. L., Jacoby R. O., Jonas A. M. Age-related and light-associated retinal changes in Fischer rats. Invest Ophthalmol Vis Sci. 1978 Jul;17(7):634–638. [PubMed] [Google Scholar]
- Lam K. W., Lee P. F. Analysis of ascorbate concentration in the aqueous humor by high-pressure liquid chromatography. Invest Ophthalmol. 1975 Dec;14(12):947–950. [PubMed] [Google Scholar]
- Lawwill T., Crockett S., Currier G. Retinal damage secondary to chronic light exposure, thresholds and mechanisms. Doc Ophthalmol. 1977 Dec 30;44(2):379–402. doi: 10.1007/BF00230089. [DOI] [PubMed] [Google Scholar]
- Lawwill T. Three major pathologic processes caused by light in the primate retina: a search for mechanisms. Trans Am Ophthalmol Soc. 1982;80:517–579. [PMC free article] [PubMed] [Google Scholar]
- Leibowitz H. M., Krueger D. E., Maunder L. R., Milton R. C., Kini M. M., Kahn H. A., Nickerson R. J., Pool J., Colton T. L., Ganley J. P. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980 May-Jun;24(Suppl):335–610. [PubMed] [Google Scholar]
- Li Z. Y., Tso M. O., Wang H. M., Organisciak D. T. Amelioration of photic injury in rat retina by ascorbic acid: a histopathologic study. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1589–1598. [PubMed] [Google Scholar]
- MASON H. S., INGRAM D. J., ALLEN B. The free radical property of melanins. Arch Biochem Biophys. 1960 Feb;86:225–230. doi: 10.1016/0003-9861(60)90409-4. [DOI] [PubMed] [Google Scholar]
- MacFaul P. A. Visual prognosis after solar retinopathy. Br J Ophthalmol. 1969 Aug;53(8):534–541. doi: 10.1136/bjo.53.8.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. R., Irvine A. R. Light-induced maculopathy from the operating microscope in extracapsular cataract extraction and intraocular lens implantation. Ophthalmology. 1983 Aug;90(8):945–951. doi: 10.1016/s0161-6420(83)80022-0. [DOI] [PubMed] [Google Scholar]
- Messner K. H., Maisels M. J., Leure-DuPree A. E. Phototoxicity to the newborn primate retina. Invest Ophthalmol Vis Sci. 1978 Feb;17(2):178–182. [PubMed] [Google Scholar]
- Naash M. I., Anderson R. E. Characterization of glutathione peroxidase in frog retina. Curr Eye Res. 1984 Nov;3(11):1299–1304. doi: 10.3109/02713688409007416. [DOI] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975 Mar 17;63(2):463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Noell W. K., Albrecht R. Irreversible effects on visible light on the retina: role of vitamin A. Science. 1971 Apr 2;172(3978):76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- Noell W. K. Effects of environmental lighting and dietary vitamin A on the vulnerability of the retina to light damage. Photochem Photobiol. 1979 Apr;29(4):717–723. doi: 10.1111/j.1751-1097.1979.tb07756.x. [DOI] [PubMed] [Google Scholar]
- Noell W. K. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 1980;20(12):1163–1171. doi: 10.1016/0042-6989(80)90055-3. [DOI] [PubMed] [Google Scholar]
- Noell W. K., Walker V. S., Kang B. S., Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966 Oct;5(5):450–473. [PubMed] [Google Scholar]
- Noell W. K., Walker V. S., Kang B. S., Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966 Oct;5(5):450–473. [PubMed] [Google Scholar]
- O'Steen W. K., Kraeer S. L. Effects of hypophysectomy, pituitary gland homogenates and transplants, and prolactin on photoreceptor destruction. Invest Ophthalmol Vis Sci. 1977 Oct;16(10):940–946. [PubMed] [Google Scholar]
- Olney J. W. Glutaate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion. J Neuropathol Exp Neurol. 1969 Jul;28(3):455–474. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- Organisciak D. T., Wang H. M., Kou A. L. Ascorbate and glutathione levels in the developing normal and dystrophic rat retina: effect of intense light exposure. Curr Eye Res. 1984 Jan;3(1):257–267. doi: 10.3109/02713688408997208. [DOI] [PubMed] [Google Scholar]
- Organisciak D. T., Wang H. M., Li Z. Y., Tso M. O. The protective effect of ascorbate in retinal light damage of rats. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1580–1588. [PubMed] [Google Scholar]
- Paetkau M. E., Boyd T. A., Grace M., Bach-Mills J., Winship B. Senile disciform macular degeneration and smoking. Can J Ophthalmol. 1978 Apr;13(2):67–71. [PubMed] [Google Scholar]
- Penner R., McNair J. N. Eclipse blindness. Report of an epidemic in the military population of Hawaii. Am J Ophthalmol. 1966 Jun;61(6):1452–1457. [PubMed] [Google Scholar]
- Perez H. D., Weksler B. B., Goldstein I. M. Generation of a chemotactic lipid from a arachidonic acid by exposure to a superoxide-generating system. Inflammation. 1980 Sep;4(3):313–328. doi: 10.1007/BF00915032. [DOI] [PubMed] [Google Scholar]
- Pfister R. R., Hayes S. A., Paterson C. A. The influence of parenteral ascorbate on the strength of corneal wounds. Invest Ophthalmol Vis Sci. 1981 Jul;21(1 Pt 1):80–86. [PubMed] [Google Scholar]
- Pfister R. R., Paterson C. A. Ascorbic acid in the treatment of alkali burns of the eye. Ophthalmology. 1980 Oct;87(10):1050–1057. doi: 10.1016/s0161-6420(80)35126-9. [DOI] [PubMed] [Google Scholar]
- Rapp L. M., Williams T. P. The role of ocular pigmentation in protecting against retinal light damage. Vision Res. 1980;20(12):1127–1131. doi: 10.1016/0042-6989(80)90050-4. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Feldman R. B. Photic retinopathy from the operating room microscope. Am J Ophthalmol. 1986 May 15;101(5):561–569. doi: 10.1016/0002-9394(86)90946-3. [DOI] [PubMed] [Google Scholar]
- Sadun A. C., Sadun A. A., Sadun L. A. Solar retinopathy. A biophysical analysis. Arch Ophthalmol. 1984 Oct;102(10):1510–1512. doi: 10.1001/archopht.1984.01040031230024. [DOI] [PubMed] [Google Scholar]
- Santos-Anderson R. M., Tso M. O., Vainisi S. J. Heredofamilial retinal dystrophy in Guinea baboons. II. Electron microscopic observations. Arch Ophthalmol. 1983 Nov;101(11):1762–1770. doi: 10.1001/archopht.1983.01040020764021. [DOI] [PubMed] [Google Scholar]
- Sperling H. G., Johnson C., Harwerth R. S. Differential spectral photic damage to primate cones. Vision Res. 1980;20(12):1117–1125. doi: 10.1016/0042-6989(80)90049-8. [DOI] [PubMed] [Google Scholar]
- Stone W. L., Katz M. L., Lurie M., Marmor M. F., Dratz E. A. Effects of dietary vitamin E and selenium on light damage to the rat retina. Photochem Photobiol. 1979 Apr;29(4):725–730. doi: 10.1111/j.1751-1097.1979.tb07757.x. [DOI] [PubMed] [Google Scholar]
- Sykes S. M., Robison W. G., Jr, Waxler M., Kuwabara T. Damage to the monkey retina by broad-spectrum fluorescent light. Invest Ophthalmol Vis Sci. 1981 Apr;20(4):425–434. [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K., Laiho K. U., Osornio A. R., Mergner W. J., Smith M. W. The role of calcium in cell injury. A review. Scan Electron Microsc. 1980;(Pt 2):437-62, 492. [PubMed] [Google Scholar]
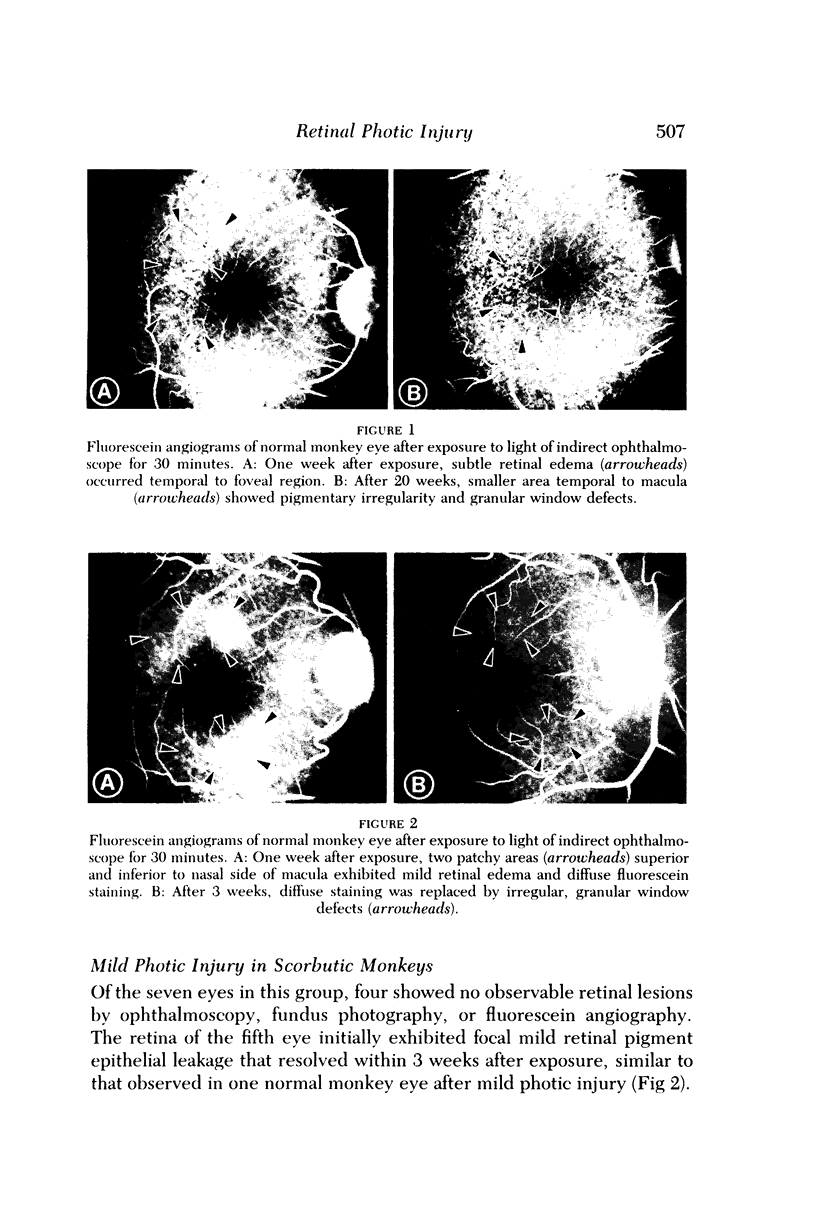
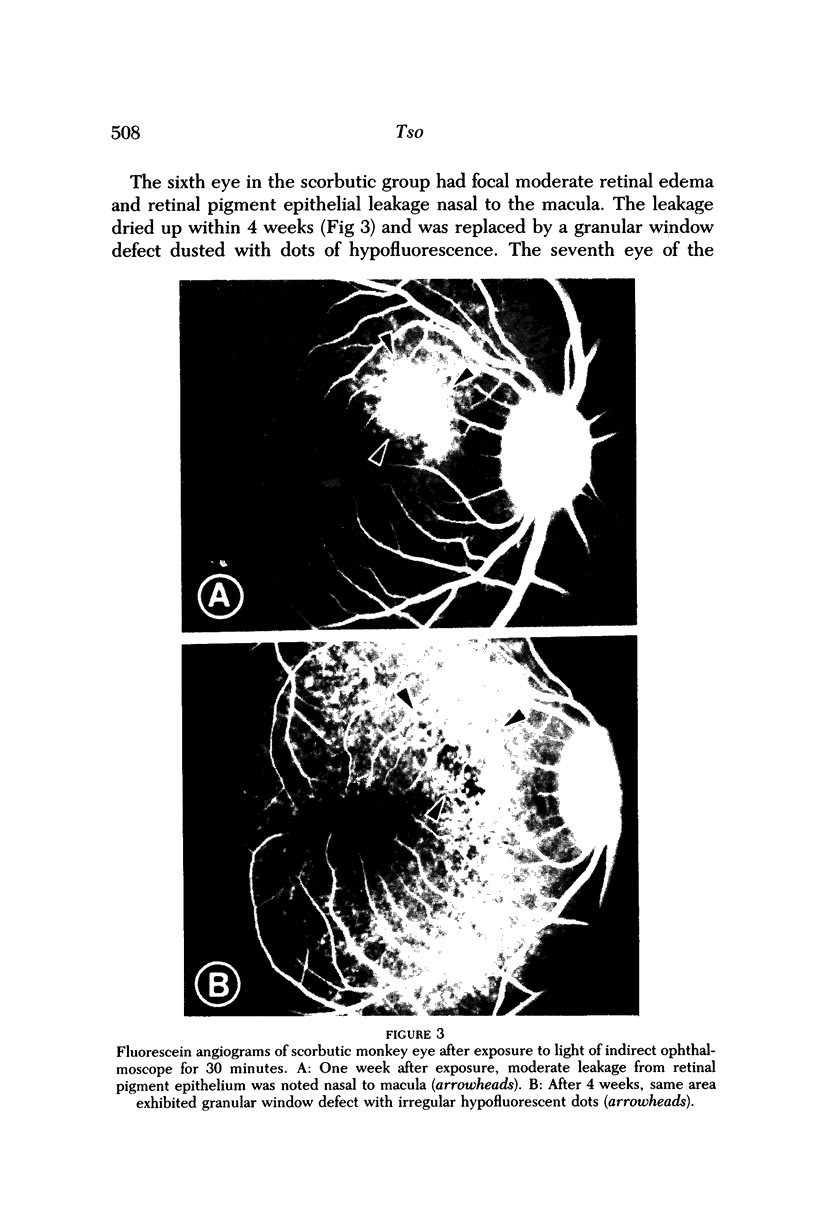
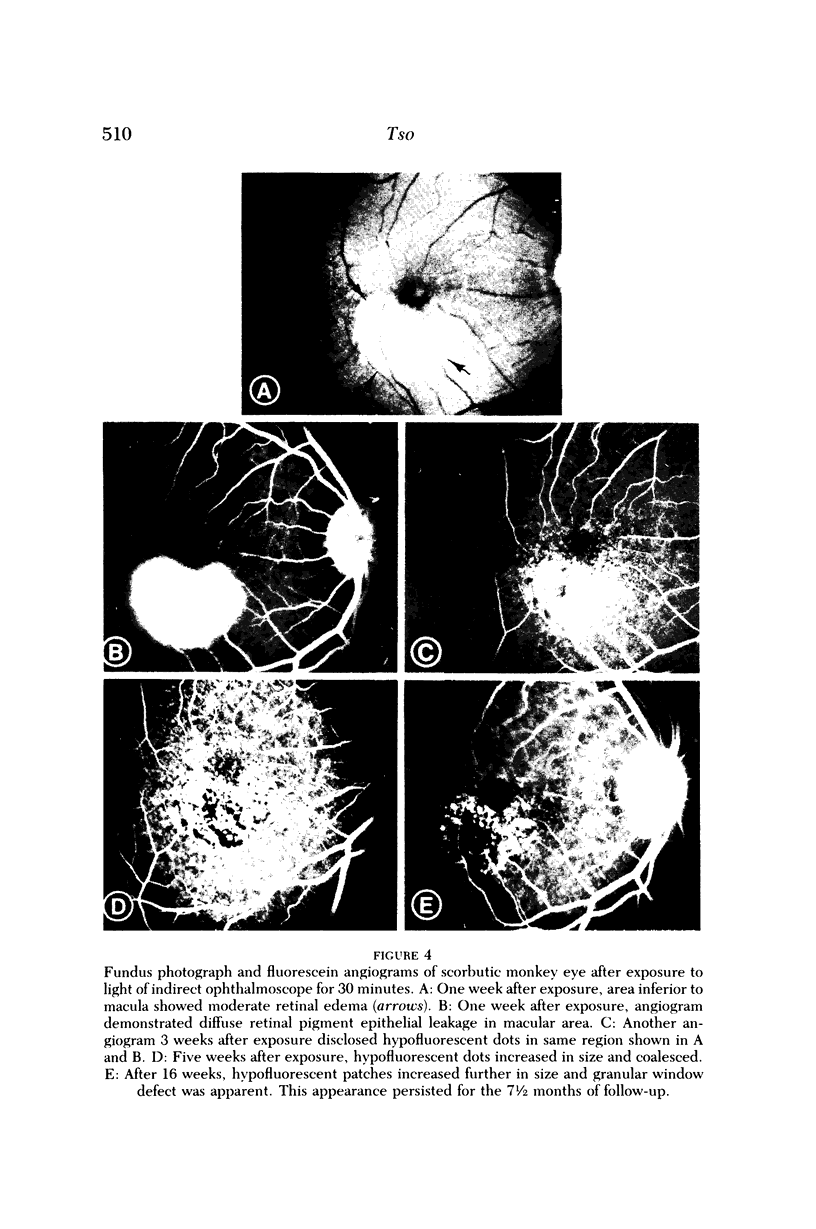
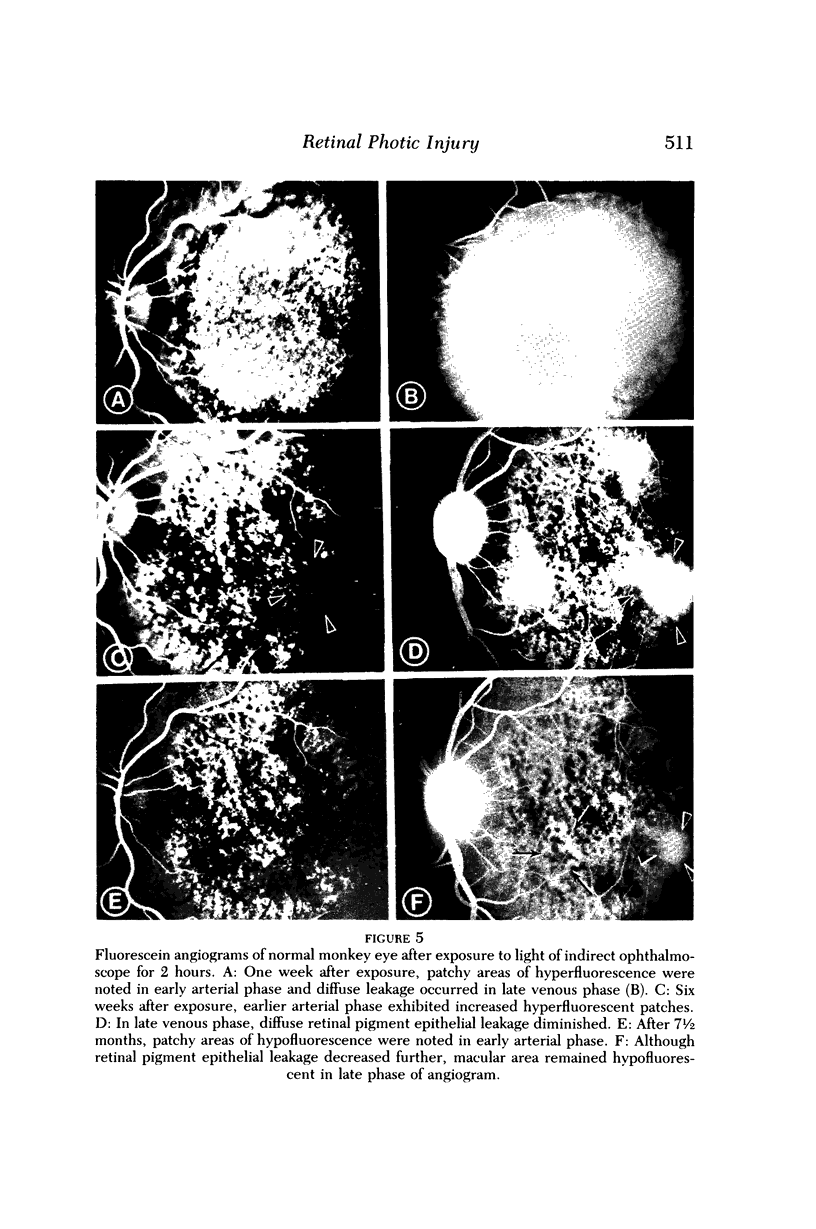
- Ts'o M. O., Fine B. S., Zimmerman L. E. Photic maculopathy produced by the indirect ophthalmoscope. 1. Clinical and histopathologic study. Am J Ophthalmol. 1972 May;73(5):686–699. doi: 10.1016/0002-9394(72)90386-8. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Cunha-Vaz J. G., Shih C. Y., Jones C. W. Clinicopathologic study of blood-retinal barrier in experimental diabetes mellitus. Arch Ophthalmol. 1980 Nov;98(11):2032–2040. doi: 10.1001/archopht.1980.01020040884020. [DOI] [PubMed] [Google Scholar]
- Tso M. O. Photic maculopathy in rhesus monkey. A light and electron microscopic study. Invest Ophthalmol. 1973 Jan;12(1):17–34. [PubMed] [Google Scholar]
- Tso M. O., Robbins D. O., Zimmerman L. E. Photic maculopathy. A study of functional and pathologic correlation. Mod Probl Ophthalmol. 1974;12(0):220–228. [PubMed] [Google Scholar]
- Tso M. O., Santos-Anderson R. M., Vainisi S. J. Heredofamilial retinal dystrophy in Guinea baboons. I. A histopathologic study. Arch Ophthalmol. 1983 Oct;101(10):1597–1603. doi: 10.1001/archopht.1983.01040020599020. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Shih C. Y. Experimental macular edema after lens extraction. Invest Ophthalmol Vis Sci. 1977 May;16(5):381–392. [PubMed] [Google Scholar]
- Tso M. O., Woodford B. J. Effect of photic injury on the retinal tissues. Ophthalmology. 1983 Aug;90(8):952–963. doi: 10.1016/s0161-6420(83)80023-2. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Woodford B. J., Lam K. W. Distribution of ascorbate in normal primate retina and after photic injury: a biochemical, morphological correlated study. Curr Eye Res. 1984 Jan;3(1):181–191. doi: 10.3109/02713688408997200. [DOI] [PubMed] [Google Scholar]
- Varma S. D., Kumar S., Richards R. D. Light-induced damage to ocular lens cation pump: prevention by vitamin C. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3504–3506. doi: 10.1073/pnas.76.7.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. D., Srivastava V. K., Richards R. D. Photoperoxidation in lens and cataract formation: preventive role of superoxide dismutase, catalase and vitamin C. Ophthalmic Res. 1982;14(3):167–175. doi: 10.1159/000265189. [DOI] [PubMed] [Google Scholar]
- Weiter J. J., Delori F. C., Wing G. L., Fitch K. A. Relationship of senile macular degeneration to ocular pigmentation. Am J Ophthalmol. 1985 Feb 15;99(2):185–187. doi: 10.1016/0002-9394(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Wing G. L., Blanchard G. C., Weiter J. J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978 Jul;17(7):601–607. [PubMed] [Google Scholar]
- Woodford B. J., Tso M. O., Lam K. W. Reduced and oxidized ascorbates in guinea pig retina under normal and light-exposed conditions. Invest Ophthalmol Vis Sci. 1983 Jul;24(7):862–867. [PubMed] [Google Scholar]
- van Heuven W. A., Lam K. W., Ray G. S. Source of subretinal fluid on the basis of ascorbate analyses. Arch Ophthalmol. 1982 Jun;100(6):976–978. doi: 10.1001/archopht.1982.01030030984017. [DOI] [PubMed] [Google Scholar]