Figure 3.
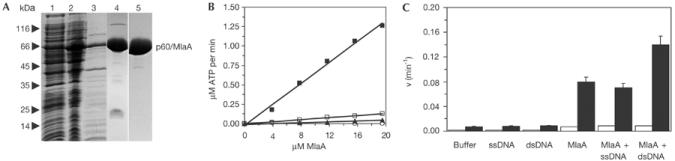
Purification and biochemical characterization of MlaA. (A) SDS–PAGE analysis (Coomassie stained) of P. abyssii MlaA expression and purification. Lane 1: insoluble E. coli fraction; lane 2: soluble E. coli fraction; lane 3: soluble fraction after heat denaturation; lane 4: MlaA after Source Q ion exchange chromatography; lane 5: MlaA after gel filtration chromatography. (B) ATPase activity assays of MlaA using TLC. The ATPase activity is greatly enhanced at 60°C (filled squares) compared to 30°C (open squares) consistent with the hyperthermophilic source organism (P. abyssii). Lys169Leu (open circles) and Glu408Gln (filled triangles) mutations dramatically reduce ATPase activity and verify MlaA Walker A and B motifs. (C) TLC ATPase assays of MlaA in the presence or absence of a twofold excess of ssDNA or dsDNA reveal that ATPase activity is stimulated twofold by dsDNA but not by ssDNA at 55°C (filled bars). At 30°C, only weak activity is observed (open bars). The rate of ATP hydrolysis per MlaA active site is shown. All assays were performed in triplicate (error bars).
