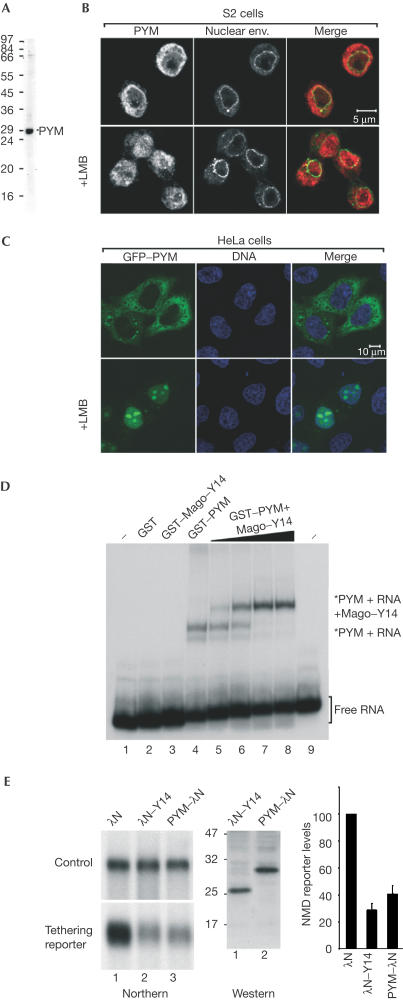
Figure 5.
PYM is a cytosolic RNA-binding protein involved in NMD. (A) Western blot with purified anti-Dm PYM antibody showing that a unique band of the right size is recognized in S2 cell extracts. (B) Immunolocalization of endogenous PYM in S2 cells. Endogenous PYM (red) is detected predominantly in the cytoplasm in untreated S2 cells, and in both the nucleoplasm and cytoplasm in cells treated for 12 h with leptomycin B (LMB). The nuclear envelope is stained with fluorescently labelled wheat germ agglutinin (WGA, green). (C) Subcellular localization of human PYM. HeLa cells were transfected with a plasmid expressing GFP–PYM. The fusion protein (green) is detected throughout the cytoplasm and excluded from the nucleoplasm. Following LMB treatment (3 h), GFP–PYM accumulates within the nucleus and the nucleolus. DNA is stained with Hoechst (blue). (D) Gel-mobility assay performed with a labelled RNA probe and the purified recombinant proteins indicated above the lanes. Dm Mago and PYM were N-terminally fused to GST. In lanes 2–4, the concentration of the recombinant proteins was 0.1 μg/μl. Lanes 5–8 show binding reactions with 0.1 μg/μl of GST–PYM supplemented with 0.005, 0.02, 0.05 and 0.1 μg/μl of GST Dm Mago–Y14 dimers, respectively. The position of the free RNA probe (lanes 1 and 9) and of the RNA-bound complexes is shown on the right. (E) NMD assay. Tethering PYM–λN to the 3′UTR of a reporter mRNA results in degradation of the reporter to a similar extent as observed when λN–Y14 is tethered. The steady-state levels of the NMD reporter in the presence of PYM or Y14 were quantified in three independent experiments, normalized to those of the transfection control and expressed as a percentage of the levels observed when the λN-peptide alone was coexpressed. The expression levels of the proteins were analysed by western blot with an anti-λN antibody.