Summary
Symposium on Membrane Trafficking in Plants
Keywords: plant cells, endomembranes, regulation, dynamics, secretion/endocytosis
Introduction
In August 2003, the plant endomembrane community gathered under the auspices of the Society for Experimental Biology to consider the latest developments in the rapidly growing field of the plant secretory pathway. The meeting was preceded by the two-day annual gathering of the European Plant Secretory Pathway Group, during which postgraduates and postdocs presented their latest data.

The Society for Experimental Biology symposium on Membrane Trafficking in Plants took place in Glasgow between 23 and 26 August 2003 and was organized by M. Blatt and G. Thiel.
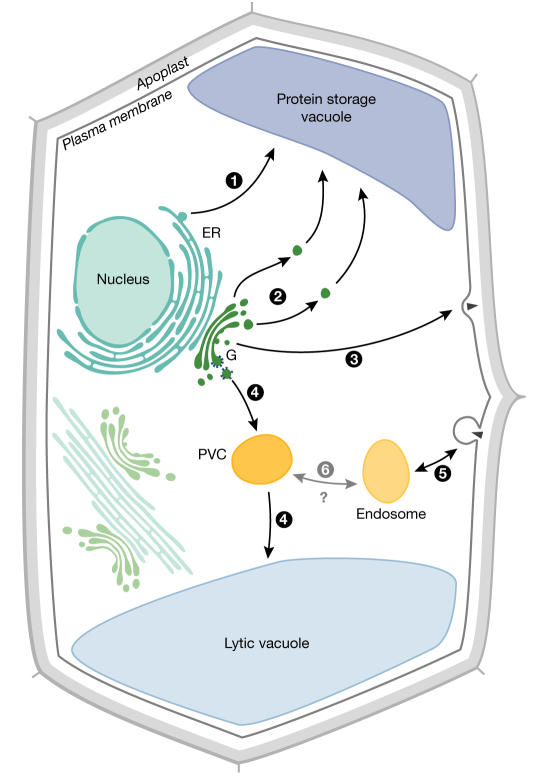
Plants have homologues of the majority of the vertebrate and yeast genes that encode both the structural and the regulatory proteins of the secretory pathway. However, it is clear that plant cells are different in (1) the organization and operation of the components of the pathway, such as highly motile Golgi stacks, (2) the necessity to sort proteins into different vacuoles and (3) the need to organize a precisely orientated apparatus for cell division (Fig 1). Here we consider the highlights of the meeting and the controversies surrounding the mechanisms by which the plant cell exports material from the site of synthesis to the site of action and imports material from the external environment.
Figure 1.
A simplified map of the secretory pathway of higher plants. Cargo synthesized and processed in the endoplasmic reticulum (ER) is transferred directly to the closely apposed and mobile Golgi apparatus (G). However, direct transfer of some storage proteins to the vacuole may occur (route 1). Proteins destined for the protein storage vacuole may leave the Golgi at the cis- and trans-compartments (route 2), whilst exocytotic cargo predominantly leaves at the trans-face (route 3). Product destined for the lytic vacuole is sorted at the trans-Golgi and routed in a clathrin- and receptor-dependent fashion through a prevacuolar compartment (PVC, route 4). Endocytosed material and recycling plasma membrane proteins are internalized into an endosome-like compartment (route 5), which might transfer material to, fuse with or mature into the PVC (route 6). The exact role of vesicles in the transfer between putative endosomes, PVCs and a lytic vacuole is unclear.
The diversity of subjects covered means that we cannot report on all of the talks in the detail they deserve. Rather, we have concentrated on emerging themes and on areas in which there have been significant and exciting advances. Our apologies to all those excellent speakers whose contributions are not discussed due to space constraints.
Molecular interactions and exit from the ER
The field of plant endomembrane biology is growing rapidly in many directions. We are no longer discussing just the discovery of molecular regulators and the characterization of component structures. In recent studies of the secretory pathways, we investigate molecular interactions and their influence on transport pathways.
The first presentation concerned endoplasmic reticulum (ER). A. Vitale (Milan, Italy) addressed the issue of the sequences that are recognized in vivo by BiP, a major ER chaperone that promotes protein folding. From studies using synthetic peptides, it is known that BiP binds to the hydrophobic amino acids that are exposed in folding and assembly intermediates, and in defective proteins. To investigate the in vivo BiP-binding partners, Vitale used an untagged homotrimeric storage protein phaseolin, derivatives of phaseolin fused to the green fluorescent protein (GFP) and secretory forms of GFP either retained in the ER due to the addition of a carboxy (C)-terminal HDEL motif or targeted to the vacuole with the phaseolin-sorting signal AFVY. Monomers of phaseolin associate with BiP, but the trimerization of phaseolin abolishes this interaction. This is because BiP binds to one of the two α-helical regions of phaseolin that is in contact with other monomers in the trimer and therefore, during the synthesis of phaseolin, association with BiP may compete with formation of the trimer. Vitale also showed that another internal region of contact between the monomers is necessary for phaseolin assembly in vivo and that it contains one potential BiP-binding site. BiP contains a C-terminal HDEL motif that retains the chaperone in the ER. J. Denecke (Leeds, UK) introduced a new concept: that BiP is transported to the vacuole when HDEL-mediated retention fails. Through systematic deletion analysis and transplantation experiments, he identified the vacuolar sorting signal of BiP and showed that it contains the predicted features of a ligand that binds to the vacuolar sorting receptor BP80. Denecke speculated that this signal could be important for targeting misfolded proteins for degradation. It is not known how the ER quality control machinery distinguishes between folding intermediates and those proteins that are permanently unfolded. The duration of the interaction of BiP with potential ligands may be the key to answering this question. During the unfolded protein response and ER stress, persistent binding of unfolded proteins to the chaperone could simply result in transport of the whole complex to the vacuole, as the HDEL-mediated retrieval system is saturable and is likely to be stretched to the limit at these times. Denecke also discussed his in vivo characterization of BP80 dynamics and the inhibition of BP80 function. Using wortmannin and a fusion of the transmembrane domain and the cytosolic tail of the receptor with GFP, he showed that recycling of BP80 between the prevacuolar compartment and the Golgi apparatus is a saturable pathway.
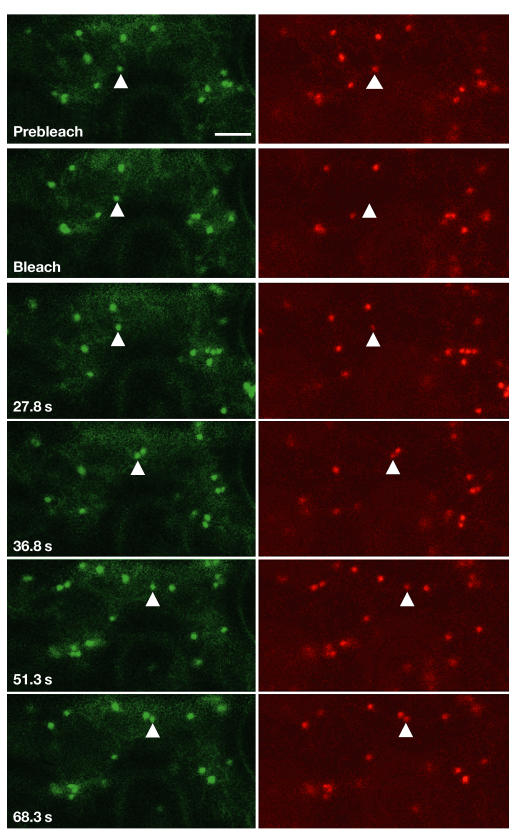
Exactly how and where proteins leave the ER for the Golgi apparatus is still an open question, which two groups addressed. By expressing Sec13 (a COPII coat protein)–GFP under the control of an inducible promoter in tobacco BY2 cells, D. Robinson (Heidelberg, Germany) showed that Sec13 punctate structures, interpreted as ER exit sites, colocalized with 50% of immunolabelled GTPase Sar1-containing structures. As Sar1 labelling rarely colocalized with the Golgi, Robinson suggested that the Golgi must move over the ER to search for exit sites. In contrast to this, F. Brandizzi (Oxford, UK) proposed a new concept of a motile export-competent complex, in which the Golgi stack and the ER export site form a mobile secretory unit. She used a photobleaching technique based on the bleaching of one fluorochrome (yellow fluorescent protein (YFP)) on a Golgi stack labelled with both cyan fluorescent protein (CFP) and YFP, and followed the recovery of fluorescence on the organelle that was still visible through the CFP channel (Brandizzi et al, 2002). She showed that Golgi bodies moving over the cortical ER network collect membrane cargo without stopping (Fig 2). Furthermore, wild-type Sar1 and a GTP-locked mutant colocalized at the Golgi area, supporting her model in which COPII structures and exit sites would be associated within the Golgi area at all times. Whether the differences in the two studies reported here simply reflect two different experimental systems has yet to be investigated.
Figure 2.
Tobacco leaf epidermal cells expressing two Golgi markers, ERD2–YFP (pseudocoloured red) and ST–CFP (pseudocoloured green), show the two proteins localizing to the same Golgi body. Photobleaching of the ERD2–YFP results in loss of fluorescence of this marker, but not the ST–CFP, followed by recovery of the signal in the same Golgi body over the time period indicated. This recovery indicates that cycling ERD2–YFP protein can transport into moving Golgi bodies. Time is expressed in seconds in individual panels. (Figure provided by FB.)
Transport to the plasma membrane and endocytosis
A hallmark of all eukaryotic cells is the compartmentalization of the secretory system into organelles that host specific biochemical reactions, whilst still enabling communication between these compartments through vesicles or tubules or by direct connections. This is achieved by loading a specific cargo into these vectors, and by tightly regulating the interactions between donor and acceptor membranes. The machinery involved in this regulation involves different groups of proteins such as the SNAREs. These are ubiquitous membrane proteins that are required for sorting vesicles correctly and for their subsequent fusion to the acceptor membrane.
Despite the fact that most of the molecular machinery needed for endocytosis in plants was described several years ago (Hawes et al, 1991), the probes and physiological markers needed to dissect functionally the endocytic pathway have only recently become available. There has been a resurgence of interest in the pathway with, for example, the study of the endocytosis and recycling of the auxin efflux carrier pin-formed (PIN1) to the plasma membrane (PM; Geldner et al, 2001) and the use of the FM family of styryl lipophilic dyes as reporters of endocytic compartments.
U. Homann (Darmstadt, Germany) used patch-clamp measurements to investigate the osmotically driven swelling and shrinkage of guard-cell protoplasts. She reported interesting data showing that changes in the PM surface area are accomplished by fusion and fission of small exo- and endocytic vesicles and not by stretching of the PM. By expressing GFP constructs of the inward K+ channel KAT 1 in guard-cell protoplasts, Homann showed that pressure-driven increases in the PM surface area induce the exocytosis of KAT 1-containing vesicles. In unstimulated turgid guard cells, KAT 1-containing vesicles colocalize with the endocytic marker FM4-64, suggesting a cycling of K+ channels at the PM.
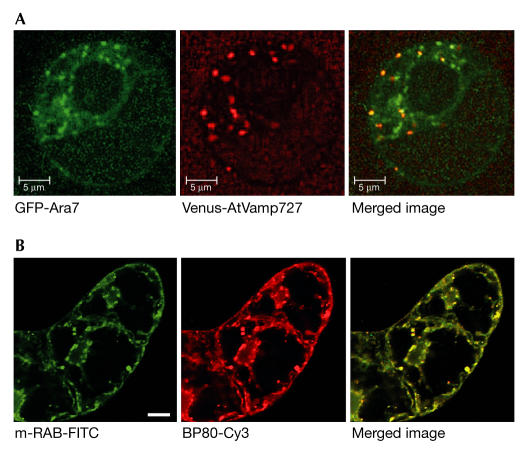
Two presentations at the meeting offered conflicting data on both the location and function of Rab5-GTPase homologues. These are involved in transport in the early mammalian endosomal pathway. T. Ueda (Wako, Japan) discussed studies involving fluorescently tagged versions of two Arabidopsis Rab5 homologues, Ara6 and Ara7. Ara6 is a member of a novel class of plant Rabs that lacks the C-terminal region for membrane attachment and is uniquely modified at the amino (N)-terminus for N-myristoylation and palmitoylation (Ueda et al, 2001). Ara7 is a conventional Rab5 homologue. In Arabidopsis protoplasts, Ara7 that is fused to the red fluorescent protein localizes on putative endosomal compartments and with a putative endosomal SNARE, AtVAMP727 (Fig 3A). Ara6–GFP colocalizes with the marker AtPEP12, a prevacuolar t-SNARE. In cells expressing a mutant GNOM (see following), Ara7 localizes to a deformed membrane along with AtVAMP727. GNOM is a guanine nucleotide exchange factor for the ADP-ribosylation factor family of small GTPases, and is involved in recycling PM proteins to an endosomal-like compartment (Geldner et al, 2003). Ueda proposed that the two Rab proteins mark a continuum in the development of a pathway from an endocytic vesicle, to an endosomal compartment and through to the prevacuole, and that Rabs regulate membrane fusion along this pathway.
Figure 3.
The endosomal/prevacuolar compartment. (A) Colocalization of Ara7 and AtVamp727 in Arabidopsis protoplasts (courtesy of T. Ueda). (B) Immunofluorescence of m-Rabmc and BP80 showing colocalization on the prevacuolar compartment (courtesy of S. Bolte). Bar, 10 μm.
By contrast, S. Bolte (Gif-sur-Yvette, France) showed that a Rab closely related to Ara6 and isolated from Mesembryanthemum crystallinum (m-Rabmc), which also has the unique N-terminal myristoylation site, is involved only in vacuolar transport (Bolte et al, 2003). In BY2 cells and Arabidopsis protoplasts, this Rab colocalizes with the prevacuolar compartment marker BP80 (Fig 3B) and partially with the Golgi. Its role in vacuolar transport was shown by the expression of a non-functional mutant, which resulted in the blockage of the lytic vacuole-targeted protein aleurain-GFP.
It is obvious that more work is needed to clarify the roles of these closely related Rabs.
Insights into protein sorting by lipid domains
The maintenance of organelle function in multi-compartmentalized cells requires specific protein sorting and delivery mechanisms to ensure efficient movement of proteins to their respective destinations. The machinery dedicated to these processes is often considered to be purely protein-based and, accordingly, the role of lipids has been ignored. However, two talks proposed that the restricted location and sorting of proteins might also be attributable to the specific lipid composition of cell membranes. G. Hinz (Göttingen, Germany) reported on the sorting of vacuolar storage proteins in the Golgi. Developing pea cotyledons contain protein storage vacuoles (PSVs) and lytic vacuoles, and initial observations suggested that the spatial separation of vacuolar sorting events takes place in the Golgi (Hillmer et al, 2001). Lumenal and membrane proteins of the PSVs exit the Golgi in dense vesicles (DVs) rather than in clathrin-coated vesicles. The Golgi apparatus works as an efficient protein sorter by separating precisely proteins destined for the ER, the PM and different types of vacuoles. Hinz proposed that major storage proteins segregate as early as the cis-Golgi and that intra-cisternal lipid domains could explain how the Golgi apparatus minimizes mis-sorting. To test this hypothesis, Hinz used prolegumins, which are transported in DVs to the PSV by a 53 kDa protein. He postulated that lipid domains could be the vectors that transport the 53 kDa protein and that they might form the DVs. Such domains could exclude other secretory proteins from DVs, thus ensuring an early and precise segregation of proteins destined for the PSV.
P. Dupree (Cambridge, UK) introduced PM lipid rafts and suggested that these might be involved in defining different areas of the surface of polarized cells. The PM is enriched in glycosylphosphatidylinositol (GPI)-anchored proteins (GAPs), and this modification represents an alternative means of attaching a protein to a membrane. It might be used to direct a specific subset of proteins to the cell surface for remodelling of the extracellular matrix and for signalling. The C-terminus of a GAP is covalently attached through phosphoethanolamine and a conserved glycan to phosphatidylinositol or to a ceramide. GAPs are enriched in the sphingolipid- and cholesterol-enriched domains (lipid rafts) in mammalian cells (Harder et al, 1998) and possibly in plant cells (Peskan et al, 2000). These proteins might be important in the functions of the cell surface, in remodelling and in cell polarization. Dupree showed that the fusion of the reporter protein, phosphinothricin acetyl transferase (PAT), to the hydrophobic C-terminal domain of an Arabidopsis GAP was sufficient to direct it to the PM. Interestingly, the protein associated with detergent-resistant membranes, indicating the presence of PM lipid rafts. He also searched the Arabidopsis genome for secretory proteins with no transmembrane domain, but with a hydrophobic C-terminus and a putative cleavage site that is needed for the attachment of the GPI moiety. This revealed 248 potential GAPs that corresponded to a variety of proteins, including putative receptors, proteases, oxidases, extensins and lipid-transfer proteins. A proteomic analysis subsequently identified 39 of these proteins.
Lipid-based sorting is obviously an area ripe for further research. For instance, do other components of the endomembrane system use such mechanisms?
Membrane transport, growth, development and physiology
The mechanisms by which membrane traffic integrates with other cellular functions such as mitosis and whole plant development were also addressed at the meeting. Intracellular transport has long been studied as an independent process, but it is obviously closely co-ordinated with other cellular functions in living cells. G. Jürgens (Tübingen, Germany) reviewed the advances made by his group in understanding the regulation of plant cell division. He focused on cytokinesis as a system to analyse syntaxin specificity in membrane fusion. Jürgens described the regulatory molecules of this complex process using Arabidopsis embryos as a model. The KNOLLE (KN) gene encodes a cytokinesis-specific syntaxin that localizes to the division plane and mediates cell-plate formation. He identified mutants that are defective in the regulation of the vesicle fusion machinery at the division plane and cell division mutants that do not progress beyond the very early stages of embryogenesis. For example, KEULE, an Arabidopsis gene involved in cytokinesis, and KN cooperate to promote vesicle fusion in the cell division plane and, if one or other of these proteins is mutated, unfused vesicles accumulate in the plane of cell division and important cytokinesis defects are visible and result in the death of seedlings. By rescuing mutant phenotypes with selective regulators, Jürgens highlighted the specific nature of the interaction of the molecules needed to organize cytokinesis. For example, some syntaxins such as AtSNAP33 are capable of interaction with KN but are not necessarily exclusively cytokinesis proteins, as deduced from their ubiquitous expression during the cell cycle and their presence at the PM as well as at the cell plate. To test the specificity of KN in membrane recognition and in fusion during cytokinesis, other syntaxins were tested for mediation of KN functionality when expressed under the control of KN cis-regulatory sequences (Muller et al, 2003). By proving that only one non-essential and non-cytokinesis-related syntaxin similar to KN, SYP112, was directed to the cell division plane and was able to rescue phenotypically kn mutants, the specificity of KN in regulating cytokinesis was confirmed. Other syntaxins such as the prevacuolar PEP12, and SYR1 (which colocalizes with KN and is very similar to it) were unable to rescue kn homozygous mutants. Jürgens demonstrated that syntaxins have three requirements to fine tune cytokinesis: expression at the M phase, absence of signals directing them to the membrane compartments other than the plane of cell division, and a sequence to mediate functional specificity in the process of membrane fusion.
T. Sanderfoot (St Paul, MN, USA) reviewed the functionality of plant-specific SNAREs in the Golgi/endosomal system and highlighted the importance of these molecules at an organismal level. A major group of SNAREs consists of 24 syntaxins that are grouped into ten gene families in Arabidopsis, each group containing 1–5 paralogous members. They are all essential although their abundance and the high degree of sequence similarity between the gene family members do not reflect any redundancy. For example, disruption of individual syntaxins from the SYP2 and SYP4 gene families is lethal, indicating an essential and unique function of each of these families. Similarly, F. Assaad (Stanford, CA, USA) illustrated the importance of SNAREs in the response of plants to abiotic and biotic stresses. For example, SYP121 and SYP122 are essential genes which, despite the similarity in their sequences, are differentially regulated on biotic stress in the host–pathogen interaction. M. Kalde (Norwich, UK) also suggested that a regulatory link might exist between elicitor-induced calcium fluxes and a rapid phosphorylation of SYP122. This may have a direct effect on the response by the host to an attack by a pathogen, a response that is mediated by the exocytosis of defence-related compounds.
New tools in microscopy
The ability to witness the dynamics of the secretory pathway in vivo is redefining our concept of cellular organisation and, as expected, the application of fluorescent protein technology pervaded every aspect of the meeting. For example, I. Moore (Oxford, UK) described the expression of secretory forms of GFP in a non-invasive live cell assay of secretion in Arabidopsis. The intracellular retention of a secreted GFP in a stable cell line can be used as a tool for forward and reverse genetic analysis of membrane transport and endomembrane organization. This method was used both for screening for secretory mutants through the retention of GFP in the ER and for identifying a putative role for RHD3 (ROOT HAIR DEFECTIVE) in the secretory pathway (Zheng et al, 2004).
With the latest advances in technology, we are able to follow cellular events in greater and greater detail. M. Oheim (Paris, France) explained the new technique of two-photon excitation fluorescence (2PEF) evanescent-field microscopy. Using this technique, it is now possible to track organelles and view the dynamics of single-molecules with millisecond time resolution in mono- and dual-fluorochrome imaging mode. A. Nakano (Wako, Japan) demonstrated the advantage of using the spinning Nipkow disk confocal microscope in combination with an ultrahigh-sensitivity, high-speed and high-resolution camera system. This now allows observation of the dynamic movement of small vesicles in a living yeast in real time.
Without doubt, our knowledge of the dynamics of the secretory pathway and its regulation will increase exponentially once the identity of more proteins and signals is discovered, and once the molecular interaction of these proteins is characterized. However, the undoubted advantages of the resolution offered by electron microscopy (EM) have yet to be fully harnessed by the endomembrane community. To unravel the complexities of protein distribution within many compartments, such as the putative endosomes and prevacuoles, will require faithful and reproducible immunogold labelling. We are still waiting for a great deal of new data in this area.
Conclusions
M. Blatt and G. Thiel assembled an exciting and stimulating group of speakers who described significant advances in our understanding of the mechanisms that regulate the biology of the plant secretory membranes. Overall, the meeting brought together researchers working on apparently disparate subjects but linked by the common theme of the secretory pathway. The array of techniques now being applied clearly illustrates the strength, diversity and importance of using complementary methodologies to elucidate and understand such a complex system.
Even though no single message can emerge from a meeting such as this, it is clear that the plant secretory community is establishing its independence from the mammalian and yeast worlds. The infamous comment, heard far too often in the past, that “it happens in plants as it happens in mammals and yeast” was refreshingly absent from this meeting. Has the plant secretory community finally acquired intellectual independence? We think so.


Acknowledgments
The meeting was generously supported by the SEB, the Gatsby Charitable Foundation, Carl Zeiss, Conviron and Universal Imaging. We thank various contributors for supplying us with micrographs for this report, and A. Kotzer for reading the manuscript. Finally, we thank J. Lippincott-Schwartz (Bethesda, MD, USA) for an inspirational opening address on live cell imaging and the animal secretory pathway.
References
- Bolte S, Brown S, Satiat-Jeunemaitre B ( 2004) The N-myristoylated Rab-GTPase m-Rabmc is involved in post-Golgi trafficking events to the lytic vacuole in plants. J Cell Sci (in press) [DOI] [PubMed] [Google Scholar]
- Brandizzi F, Snapp EL, Roberts AG, Lippincott-Schwartz J, Hawes C ( 2002) Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell 14: 1293–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K ( 2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G ( 2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 141–142 [DOI] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K ( 1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol, 141: 929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes CR, Coleman JOD, Evans DE ( 1991) Endocytosis, Exocytosis and Vesicle Traffic in Plants. Cambridge University Press, Cambridge, UK [Google Scholar]
- Hillmer S, Movafeghi A, Robinson DG, Hinz G ( 2001) Vacuolar storage proteins are sorted in the cis-cisternae of the pea cotyledon Golgi apparatus. J Cell Biol 152: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Wagner W, Volker A, Schellmann S, Nacry P, Kuttner F, Schwarz-Sommer Z, Mayer U, Jurgens G ( 2003) Syntaxin specificity of cytokinesis in Arabidopsis. Nat Cell Biol 5: 531–534 [DOI] [PubMed] [Google Scholar]
- Peskan T, Westermann M, Oelmuller R ( 2000) Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur J Biochem, 267: 6989–6995 [DOI] [PubMed] [Google Scholar]
- Ueda T, Yamaguchi M, Uchimiya H, Nakano A ( 2001) Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J 20: 4730–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Kunst L, Hawes C, Moore I ( 2004) A GFP-based assay reveals a role for RHD3 in transport between the endoplasmic reticulum and Golgi apparatus. Plant J (in press) 37: 398–414 [DOI] [PubMed] [Google Scholar]