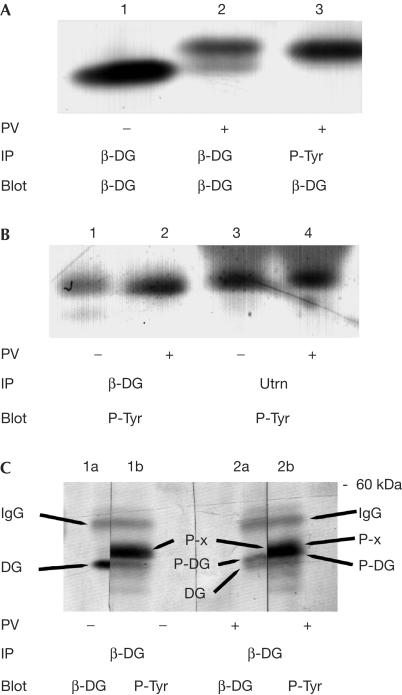
Figure 2.
Immunoprecipitation of a tyrosine phosphoprotein with β-dystroglycan and utrophin. Cell extracts from adherent HeLa cells, untreated (−) or treated (+) with peroxyvanadate (PV), were immunoprecipitated (IP) with antisera against either β-dystroglycan (β-DG), phosphotyrosine (P-Tyr) or utrophin (Utrn) followed by western blotting (Blot) with sera against either β-DG or P-Tyr. (A) Peroxyvanadate caused a reduction in electrophoretic mobility of immunoprecipitated β-DG (compare lanes 1 and 2); a band corresponding to the higher migrating species could also be immunoprecipitated with P-Tyr antiserum (lane 3). (B) β-DG and utrophin are both able to immunoprecipitate a band of ∼45 kDa from HeLa cell extracts, which is recognized by antiphosphotyrosine sera, whether the cells were first treated with PV or not. (C) Untreated HeLa cell extracts (−) or PV-treated extracts (+) were immunoprecipitated with β-DG, separated by SDS–PAGE, transferred to PVDF and each single gel lane was cut in half. Each half lane was probed with either β-DG or P-Tyr antisera and developed as above. Cut halves were re-aligned to reveal four identifiable bands: namely IgG, upper band across all lanes; and unphosphorylated β-DG (lane 1a, lower band in lane 2a); tyrosine-phosphorylated β-DG (upper band in lane 2a, lower part of main band in lane 2b); and the ∼45 kDa phosphotyrosine band (lane 1b, upper part of lane 2b), arrowed as DG, P-DG and P-x, respectively.