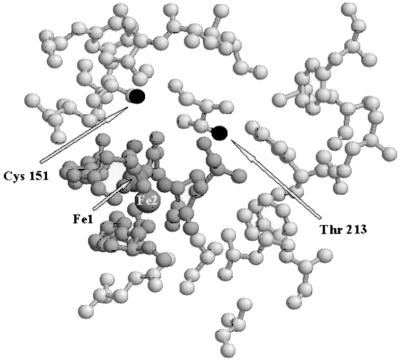
FIG. 1.
Active site of the sMMO hydroxylase based on the X-ray crystal structure (9), showing the positions of the mutated residues. The iron atoms Fe-1 and Fe-2, which constitute the binuclear iron center, are ligated by four Glu and two His residues and three solvent molecules (dark grey). They lie in a solvent-accessible cavity lined by hydrophobic residues (light grey). The side-chain Oγ and Sγ moieties of the mutated residues (Cys 151 and Thr 213) are the only nonligating protonated side-chain groups within this cavity and are shown in black.