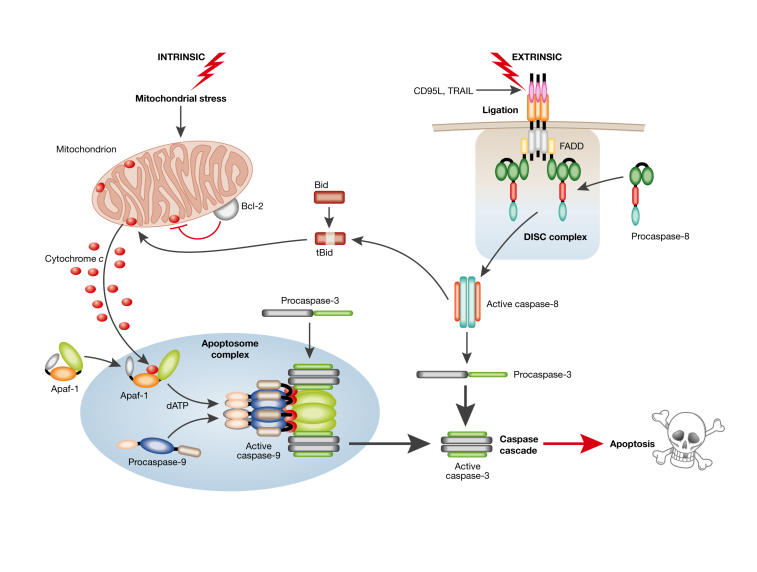
Figure 1.
Apoptosis: the 'extrinsic' and 'intrinsic' pathways to caspase activation. Two major apoptotic pathways are illustrated: one activated via death receptor activation ('extrinsic') and the other by stress-inducing stimuli ('intrinsic'). Triggering of cell surface death receptors of the tumour necrosis factor (TNF) receptor superfamily, including CD95 and TNF-related apoptosis-inducing ligand (TRAIL)-R1/-R2, results in rapid activation of the initiator caspase 8 after its recruitment to a trimerized receptor-ligand complex (DISC) through the adaptor molecule Fas-associated death domain protein (FADD). In the intrinsic pathway, stress-induced apoptosis results in perturbation of mitochondria and the ensuing release of proteins, such as cytochrome c, from the inter-mitochondrial membrane space. The release of cytochrome c, from mitochondria is regulated in part by Bcl2 family members, with anti-apoptotic (Bcl2/ Bcl-XL/Mcl1) and pro-apoptotic (Bax, Bak and tBid) members inhibiting or promoting the release, respectively. Once released, cytochrome c binds to apoptotic protease-activating factor 1 (Apaf1), which results in formation of the Apaf1–caspase 9 apoptosome complex and activation of the initiator caspase 9. The activated initiator caspases 8 and 9 then activate the effector caspases 3, 6 and 7, which are responsible for the cleavage of important cellular substrates resulting in the classical biochemical and morphological changes associated with the apoptotic phenotype (reviewed in Adams, 2003; Danial & Korsmeyer, 2004).