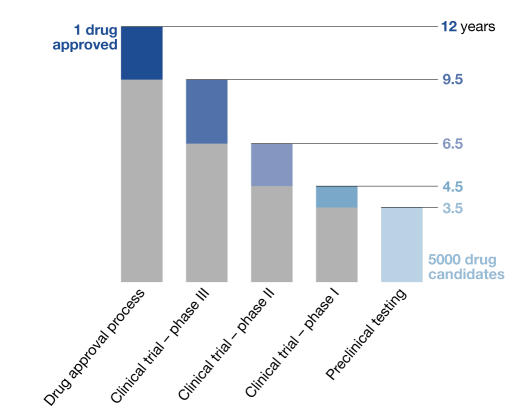
Figure 1.
Current time-scale of drug approval process. New drugs are developed through several phases: synthesis and extraction of new compounds, biological screening and pharmacological testing, pharmaceutical dosage formulation and stability testing, toxicology and safety testing, phase I, II and III clinical evaluation process, development for manufacturing and quality control, bioavailability studies and post-approval research. Before testing in humans can start, a significant body of pre-clinical data must be compiled, and appropriate toxic doses should be found for further in vivo testing to ensure human safety. Toxicology, pharmacology, metabolism and pharmaceutical sciences represent the core of pre-clinical development.