Figure 2.
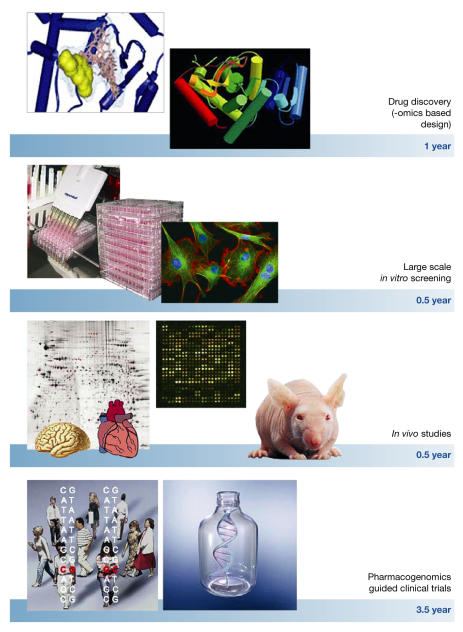
The increasing availability of quantitative biological data from the human genome project, coupled with advances in instrumentation, reagents, methodologies, bioinformatics tools and software, are transforming the ways drug discovery and drug development are performed. The ability to combine high-throughput genomic, proteomic, metabolomic and other experimental approaches with drug discovery will speed up the development of safer, more effective and better-targeted therapeutic agents. Functional genomics approaches should be exploited throughout the entire drug development process. Particularly, combinatorial chemistry, in silico structure prediction, new scaffold-like molecular weight compounds targeting conserved regions of multiple protein family members, accompanied by high-throughput X-ray crystallography and proteomic-based drug target discovery, will reduce the time required for drug discovery. Large-scale (robotics) in vitro screening using cultured human cell lines and in vivo studies on 'humanized' mouse models combined with functional genomic analysis of different organs will speed up testing. Finally, pharmacogenomics-guided clinical trials, followed by toxicogenomics-based analyses should shorten the clinical phase of testing by as much as 3–4 years.