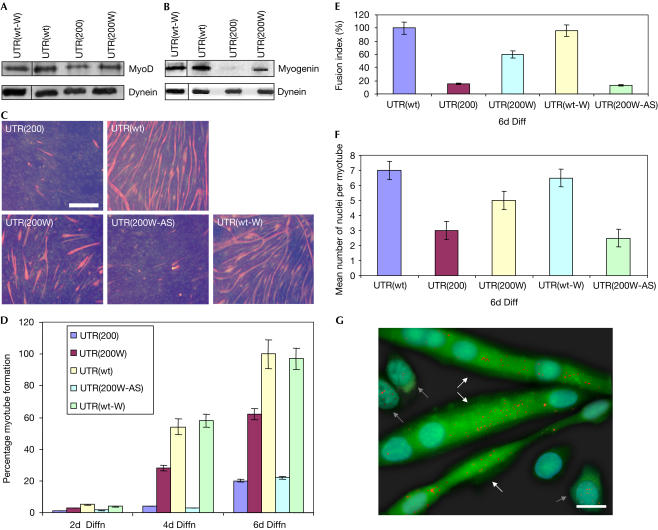
Figure 4.
Restoration of defective MyoD levels and muscle-cell differentiation. (A) Reduced MyoD levels were detected by western blotting in cells expressing the mutant DMPK 3′ UTR (UTR(200)), as compared with cells expressing the wild-type DMPK 3′ UTR (UTR(wt)) or the wild-type DMPK 3′ UTR and WPRE (UTR(wt-W)). WPRE-mediated transport of mutant DMPK 3′ UTR caused an increase in the cellular MyoD levels (UTR(200W)). (B) After 4 days of cell differentiation, myogenin levels were found to be increased, by western blotting, in cells expressing the UTR(200W) construct, as compared with cells expressing the UTR(200) construct. Normal myogenin was detected in cells that expressed UTR(wt) and UTR(wt-W). (C) Immunocytochemistry against myosin heavy chain (MHC) showed a marked reduction of myotube formation in cells expressing the mutant DMPK 3′ UTR (UTR(200)), as compared with cells expressing the wild-type DMPK 3′ UTR (UTR(wt)) or the wild-type DMPK 3′ UTR and WPRE (UTR(wt-W)), after 6 days of differentiation. Addition of WPRE downstream of the mutant DMPK 3′ UTR significantly increased myoblast differentiation (UTR(200W)) but not when it was present in its antisense form (UTR(200W-AS)). Scale bar, 0.5 mm. (D–F) Summary of MHC staining indicates that MHC levels are highly increased in differentiated UTR(200W) cells compared with UTR(200) cells, as detected by counting positively stained myotubes, the rate of fusion into myotubes and the number of nuclei per myotube. (G) RNA foci localization on differentiated cells expressing the WPRE sequence downstream of the mutant DMPK 3′ UTR, as detected by FISH, showed that transcripts/foci (shown as red dots) are mainly located in the cytoplasm in multinucleated myotubes (shown with white arrows), whereas undifferentiated myoblasts (shown with grey arrows) contain most of the RNA foci in the nucleus. Moreover, EGFP signal is significantly higher in differentiated myotubes than in undifferentiated myoblasts. Scale bar, 0.05 mm.