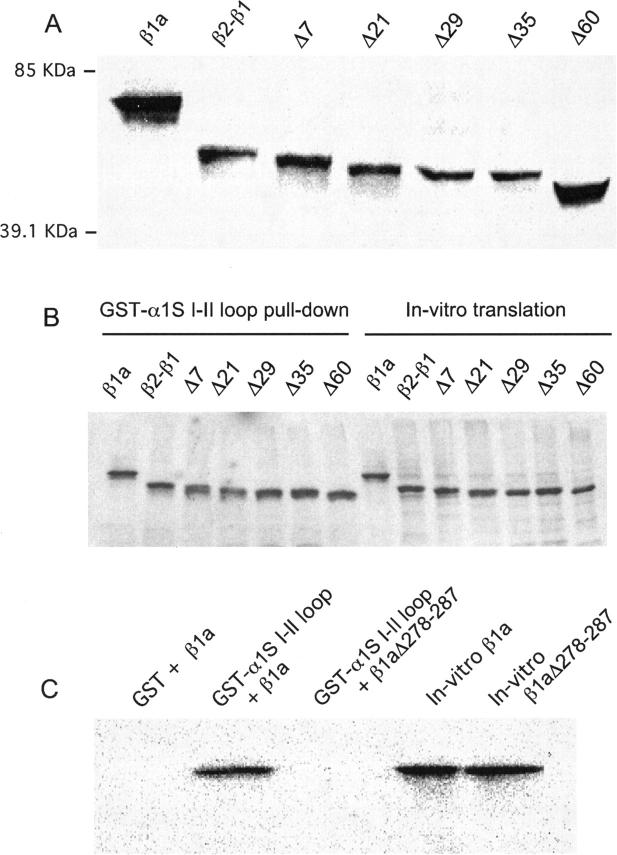
FIGURE 2.
Size of truncated β variants and interaction with the I-II loop of the α1S pore subunit. (A) Immunoblot of a 10% SDS-polyacrylamide gel of in vitro translated β variants. After transfer to nitrocellulose and incubation with anti-T7 antibody, the blot was revealed by high performance chemiluminescence. Indicated are the relative mobilities of two out of five M.W. markers run in the same gel. (B) Autoradiogram of a 15% SDS-polyacrylamide gel of in vitro translated [35S]methionine-labeled β variants pulled down by recombinant GST-α1S I-II loop fusion protein immobilized on gluthatione(GS)-Sepharose. Lanes 1–7 show pellets (pull-downs) after 2 h of incubation of GS-Sepharose/GST-α1S I-II loop and the indicated in vitro translated construct. Lanes 8–14 show equal aliquots of the in vitro translation mixture with each β variant as template. (C) Controls of the pull-down protocol are shown on an autoradiogram of a 15% SDS-polyacrylamide gel of in vitro translated [35S]methionine-labeled β variants. Lane 1 shows absence of β1a pull down by recombinant GST lacking α1S I-II loop fusion. Lane 2 shows β1a pull down by GST-α1S I-II loop fusion. Lane 3 shows absence of pull down of β1aΔ278-287 by recombinant GST-α1S I-II loop fusion. Lanes 4 and 5 show in vitro translated β1a and β1aΔ278-287.