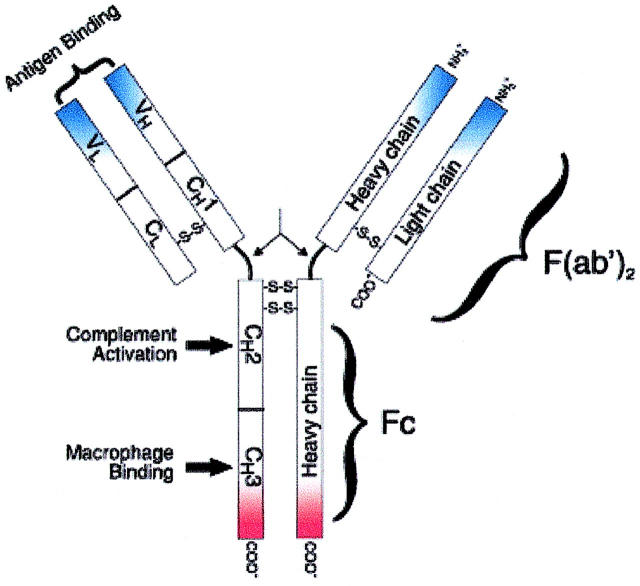
FIGURE 1.
Schematic representation of an IgG molecule. IgG (MW, 150-kDa) have a basic four-chain monomeric structure consisting of two identical heavy chains and two identical light chains. The heavy chain contains one variable domain (VH) and three constant domains (CH1, CH2, and CH3). The region between the CH1 and CH2 is the hinge region and permits flexibility between the two Fab arms of the Y-shaped antibody molecule, allowing them to open and close to accommodate binding to two antigenic determinants separated by a fixed distance. Functionally, an IgG molecule can be divided into two portions: Fragment antigen binding (Fab) fragment, which is the antigen-binding site, and Fragment crystallizable (Fc) fragment, for which Protein A has high affinity.