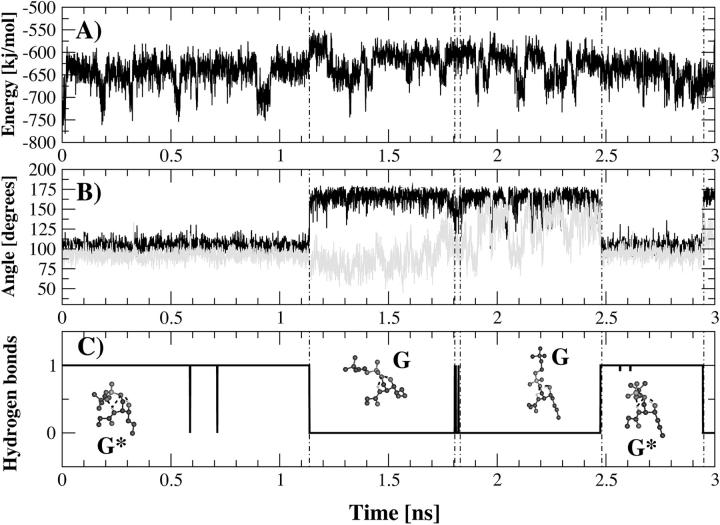
FIGURE 3.
Time course of intramolecular nonbonded energy (A), dihedral transitions (B), and hydrogen-bond formation (C) for the 3-ns simulations at 288 K of conformer G*. The analyzed dihedral angles are defined by the phosphorus atom, the oxygen atom O2, and the carbon atoms C1 and C2 (in black) and by the oxygen atom O″1, phosphorus atom, oxygen atom O1, and carbon atom C1 (in gray). The conformations that PSM adopts along the trajectory (G and G*) are shown in the lowest panel. The formation of a hydrogen bond between the NH group and the phosphate esteric oxygen atom O″1 is correlated to the dihedral angle transitions (Fig. 3 C).